Scroll to:
Triglyceride glucose index is associated with subclinical atherosclerosis and subclinical myocardial dysfunction in patients with newly diagnosed type 2 diabetes mellitus
https://doi.org/10.14341/DM13073
Abstract
BACKGROUND: Previous studies have shown that, the triglyceride glucose index (TyG index) is related with the development of cardiovascular disease.
AIM: Our novel study aimed to determine whether the TyG index measured at the time of diagnosis conducted on newly diagnosed type 2 diabetic individuals and the relationship between TyG index and carotid intima media thickness, as well as both myocardial functions and epicardial adipose tissue was investigated.
MATERIALS AND METHODS: The study included 105 individuals (58 F, 47 M; mean age 50.4±9.8 years) newly diagnosed with T2DM and 51 healthy subjects (30 females, 21 males, mean age 49.8±8.9 years) without any chronic disease as the control group. In addition to laboratory parameters, transthoracic echocardiography carotid intima-media thickness with linear vascular probe were examined in all individuals.
RESULTS: TyG index was significantly higher in newly diagnosed type 2 diabetic individuals compared to the controls. There was a positive correlation between the TyG index and carotid intima-media thickness, epicardial fat thickness, HbA1c, Homa-IR, body surface area, waist circumference, hip circumference, body mass index and CRP. When diastolic functions were considered, there was a negative correlation with E/A and a positive correlation with E/e’ septal. TyG index was also negatively correlated with EF. Regression analysis revealed that age and TyG index were associated with an increase in carotid IMT thickness.
CONCLUSION: TyG index measured at the time of diagnosis in newly diagnosed type 2 diabetic patients is also associated with subclinical atherosclerosis, deterioration in left ventricular systolic and diastolic functions.
Keywords
For citations:
Ustabas S.H., Altunoglu E.G., Karabag T. Triglyceride glucose index is associated with subclinical atherosclerosis and subclinical myocardial dysfunction in patients with newly diagnosed type 2 diabetes mellitus. Diabetes mellitus. 2024;27(3):224-232. https://doi.org/10.14341/DM13073
INTRODUCTION
Diabetes mellitus is a chronic disease with an increasing incidence. The International Diabetes Federation (IDF) reported in 2021, there were 537 million diabetic patients worldwide and this number is expected to increase to 783 million in 2045. Type 2 diabetes is the most common type of diabetes, accounting for 90% of all cases. Long-term uncontrolled hyperglycemia can damage many organs and cause many complications such as cardiovascular disease (CVD), neuropathy, nephropathy, and retinopathy [1].
Chronic complications of diabetes are grouped as microvascular complications due to damage to small vessels and macrovascular complications due to arterial damage. Macrovascular complications may occur in the form of cardiovascular disease resulting in myocardial infarction (MI), peripheral vascular disease, and cerebrovascular disease resulting in stroke [2]. Cardiovascular diseases (CVD) are the most common cause of death in both type 1 and type 2 diabetic patients. Cardiac autonomic neuropathy, microangiopathy, diabetic cardiomyopathy and especially atherosclerosis are pathogenetic factors that play a role in the development of cardiovascular disease in diabetic patients [3]. Diabetes is a major risk factor for the development of atherosclerosis. According to the Framingham heart study, diabetic patients have a 2- to 4-fold increased risk of atherosclerosis and obstructive coronary artery disease [4]. Chronic inflammation, advanced glycation end products, oxidative stress, protein kinase C activation is associated with development of atherosclerosis in diabetic patients as well as dyslipidemia, hyperglycemia [5]. With aggressive risk factor modification in diabetic patients, a significant improvement is achieved in the 10-year risk of coronary artery disease and in the mortality and morbidity of atherosclerotic cardiovascular diseases [6].
Complications are common in type 1 and type 2 diabetes and are known to be an important cause of mortality and morbidity. At least one complication is present at the time of diagnosis in 25% of type 2 diabetic individuals, and early detection of these complications is of high clinical importance. Glycemic control and clinical risk factors alone are not sufficient to predict the development of complications [7][8].
The triglyceride glucose index (TyG index), calculated via fasting glucose and triglyceride levels. It is known as a reliable predictor of insulin resistance and has been shown to be better than homeostatic model (HOMA-IR) in detecting insulin resistance. Due to its association with insulin resistance, TyG index is one of the predictive markers in the development of type 2 diabetes. It also stands out with its low cost since it does not require an additional test such as the TyG index plasma insulin level, which is calculated by the fasting glucose and fasting triglyceride level, which are routine tests in the clinic. In many studies, the TyG index was found to be positively correlated with coronary artery disease, carotid atherosclerosis, hypertension, metabolic syndrome, arterial stiffness, and coronary artery calcification [9, 10].
We think that our study is different from previous studies because it was conducted on newly diagnosed type 2 diabetic individuals and the relationship between Tyg index and carotid intima media thickness, as well as both myocardial functions and epicardial adipose tissue was investigated.
MATERIALS AND METHODS
Patient selection
A total of 156 patients (88 women, 68 men, mean age 49.8±8.9 years) were included in the study, 105 newly diagnosed diabetic and 51 non-diabetic patients with sufficient cooperation and orientation over the age of 18 who applied to the Internal Medicine outpatient clinics, and whose consent was obtained for the study.
Patients with previous use of antidiabetic, lipid-lowering and antihypertensive drugs, with a diagnosis of coronary artery disease, with severe chronic diseases such as liver cirrhosis, end-stage renal disease and malignancy, with pregnancy, additional metabolic disease, morbid obesity and type 1 diabetic were not included in the study. Individuals using drugs that directly or indirectly affect blood pressure, cardiac functions and heart rate were excluded from the study.
Detailed medical history was obtained from all patients who met the inclusion criteria and physical examinations were performed. demographic data such as body weight (in kg), height (in cm), and hip and waist circumference (in cm) were measured and recorded. Waist and hip circumference was measured while standing and arms open to the sides. Waist circumference was measured at the level of the umbilicus and at the sides of the subcostal region. Hip circumference was measured from the most protruding part of the symphysis pubis anteriorly and the gluteal region posteriorly. Heart rate, systolic-diastolic blood pressures were measured and recorded.
Laboratory parameters
Blood samples were obtained after at least 8 hours of night fasting and fasting blood glucose, laboratory tests about renal functions (urea-creatinine), liver functions (aspartate aminotransferase, alanine aminotransferase), insulin, HbA1c, uric acid, gamma glutamyl transferase (GGT), lipid panel (total cholesterol, low-density lipoprotein, triglyceride, high-density lipoprotein, C-reactive protein (CRP), hemogram and microalbumin/creatinine levels in spot urine were recorded. One blood sample was collected each into a BD (Becton Dickinson, NJ, US) vacutainer gel plastic yellow capped serum separator tube (SST) and BD vacutainer K2-Ethylene diamine tetraacetic acid (EDTA) purple capped hemogram tube. Spot urine was collected into one BD vacutainer urine tube. The SST was turned upside down 5–6 times for sufficient contact with the clot activator in its content. The samples in the hemogram tubes were turned upside down 8–10 times in order to mix them sufficiently with the anticoagulant. After waiting for at least 20 minutes for coagulation to occur, SSTs were centrifuged at 1000xg for 15 minutes in a Beckman Coulter Allegra X-15R Centrifuge (2–8 °C). The hemogram was studied on the Mindray BC-6800 Plus from the sample in the purple capped tube. HbA1c was also studied on the Arkray ADAMS™ A1c HA-8180V Analyzer using the sample in the same purple capped tube. The insulin level was studied from the sample in the STT with Roche Diagnostics, Cobas 8000 e602. Fasting blood sugar, urea, creatinine, AST, ALT, GGT, LDL, HDL, total cholesterol, triglyceride, uric acid, CRP tests and microalbumin/creatinine levels in spot urine were studied with Roche Diagnostics, Cobas 8000 c702.
Triglyceride glucose index was calculated with the formula ln [Fasting triglyceride (mg/dl) x fasting glucose (mg/dl)]/2. Homa-IR was calculated with the formula [Fasting glucose (mg/dl) x Fasting insulin (mU/L)]/405.
Echocardiographic examination
Transthoracic echocardiography was performed on the patients in the left lateral decubitus position using a 2.5–3.5 MHz transducer, Philips EPIQ 7 echocardiography device (Philips Healthcare, 3000 Minuteman Road, Andover, MA, USA). M-mode tracings were recorded at a speed of 50 mm/sec and Doppler tracings were recorded at a speed of 100 mm/sec. All measurements were made with electrocardiography. M-mode and 2-D measurements were made from the parasternal long-axis view. Diastolic functions, tissue Doppler parameters measured from the lateral, septal and tricuspid annulus, and ejection fraction with Simpson’s method were measured in the patients in addition to conventional echo parameters. Epicardial fat thickness was calculated on parasternal long-axis imaging of the anterior aspect of the right ventricle. Carotid intima-media thickness was measured 2–3 cm above the carotid bifurcation separation with a linear transducer in B mode. The average of three separate measurements was recorded after all measurements. All images were performed by a single processor to avoid differences between observers.
Statistical Analysis
Statistical analyzes were performed by using SPSS version 22.0. The conformity of the variables to the normal distribution was examined by histogram graphics and the Kolmogorov-Smirnov test. Mean, standard deviation and median values were used while presenting descriptive analyzes. Comparison of continuous variables between two independent groups was made with the independent t-test when parametric test assumptions were met and the Mann Whitney-U test when parametric test assumptions were not. Spearman correlation tests were used to analyze the measurement data with each other. P<0.05 was accepted as statistical significance. The independent effects of different predictors on IMT and epicardial fat thickness were examined using a multivariate linear regression model. Model fit was evaluated using the required residual and fit statistics. The predictors included in the model were selected among the variables found to be associated with atherosclerosis in the literature review and the variables with an r coefficient above 0.300 in the correlation analysis, and that would not affect each other.
Ethics review
The study was initiated after the approval of the local ethics committee (Saglik Bilimleri University, Istanbul Education and Research Hospital ethics committee-ID number: 2913) .
RESULTS
For the study, 105 people with newly diagnosed diabetes were included in the case group (58 F, mean age 50.4±9.8 years) and 51 healthy people without diabetes and any chronic disease were included in the control group (30 F,mean age 48.5±6.4 years). 39% of the case group and 47.1% of the control group were smokers. %48.5 of the diabetic group, %9.8 of the controls were obese (BMI≥30 kg/m2).
Demographic, anthropometric and biochemical parameters of the study population are shown in Table 1. Heart rate, systolic and diastolic blood pressures were significantly higher in the newly diagnosed type 2 diabetic group compared to the control group (Table 1). Age, gender and smoking rate were similar between the groups. In the comparison of the anthropometric measurements of the patients, body surface area, body mass index, waist circumference and hip circumference were found to be statistically significantly higher in the newly diagnosed type 2 diabetic group compared to the control group (Table 1). In the comparison of the triglyceride glucose indices of the patients, the TyG index was found to be statistically significantly higher in the newly diagnosed type 2 diabetic group (Table 1).
When the transthoracic echocardiographic measurements of the patients were compared, interventricular septum, posterior wall thicknesses, aortic and left atrium diameters were significantly higher in the diabetic group compared to the control group (Table 2). Ejection fraction, E/A ratio and diastolic myocardial velocities were significantly lower in the diabetic group than in the control group (Table 2). Septal E/e’ ratio was also significantly higher in the diabetic group compared to the control group (Table 2).
A statistically significant positive correlation between the patients’ triglyceride glucose index measurements and HbA1c and Homa-IR was found. There was a significant positive correlation between the TyG index and body surface area, waist circumference, hip circumference, body mass index and CRP (Table 3). In the correlation analysis of diastolic functions, there was a statistically significant negative correlation between triglyceride glucose index and E/A measurements. While there was a statistically significant positive correlation between triglyceride glucose index and E/e’ septal, no correlation was found between triglyceride glucose index and E/e’ lateral. A significant positive correlation between TyG index and carotid intima-media thickness and epicardial fat thickness was found (Figure 1). There was a statistically significant negative correlation between TyG index and left ventricular ejection fraction (Table 3).
In the regression analysis, it was determined that age and increase in TyG index were associated with an increase in carotid IMT thickness (table 4).
Table 1: Comparison of demographic, anthropometric and biochemical values of newly diagnosed type 2 diabetes mellitus patients and control groups
|
Newly Diagnosed |
Control (n=51) |
p |
|
|
Gender (female, n (%)) |
58 (55.2) |
30 (58.8) |
0.673 |
|
Smoking n (%) |
41 (39.0) |
24 (47.1) |
0.343 |
|
Age (year) |
50.4±9.8 |
48.5±6.4 |
0.160 |
|
Height (cm) |
165.2±8.8 |
167.3±8.6 |
0.163 |
|
Weight (kg) |
80.5±15.4 |
69.2±11.5 |
<0.001 |
|
Body Surface Area (m2) |
1.91±0.21 |
1.78±0.17 |
0.002 |
|
Body Mass Index (kg/m2) |
29.4±5.0 |
24.8±4.1 |
<0.001 |
|
Waist Circumference (cm) |
107.2±10.9 |
94.5±12.5 |
<0.001 |
|
Hip Circumference (cm) |
109.3±9.1 |
102.4±9.1 |
<0.001 |
|
Obesity (%) |
48.5 |
9.8 |
<0.001 |
|
Systolic Blood Pressure (mm/Hg) |
138.2±20.3 |
126.7±17.7 |
0.001 |
|
Diastolic Blood Pressure (mm/Hg) |
82.8±12.8 |
76.4±14.4 |
0.006 |
|
Heart Rate (/dk) |
85.0±13.5 |
80.4±9.9 |
0.019 |
|
Glucose (mg/dL) |
191.1±82 |
91.4±5.9 |
<0.001 |
|
Urea (mg/dL) |
27.3±7.7 |
27.5±8.5 |
0.908 |
|
Creatinine (mg/dL) |
0.71±0.15 |
0.73±0.17 |
0.469 |
|
eGFR (ml/dk/1.73 m2) |
102.5±13.7 |
103.9±9.6 |
0.564 |
|
AST (U/L) |
21.1±13.2 |
18.5±7.9 |
0.137 |
|
ALT (U/L) |
28.7±24.1 |
17.8±11.2 |
<0.001 |
|
Total Cholesterol (mg/dL) |
213.3±72.8 |
196.2±40.0 |
0.120 |
|
Triglyceride (mg/dL) |
209.5±169.4 |
96.6±43.1 |
<0.001 |
|
LDL (mg/dL) |
124.7±40.0 |
120.8±35.4 |
0.554 |
|
HDL (mg/dL) |
44.9±14.3 |
56.0±14.9 |
<0.001 |
|
HbA1c (%) |
9.17±2.46 |
5.36±0.25 |
<0.001 |
|
TyG Index |
5.16±0.38 |
4.49±0.23 |
<0.001 |
|
CRP (mg/L) |
6.1±6.1 |
1.7±1.5 |
<0.001 |
|
GGT(U/L) |
40.6±35.1 |
14.9±8.7 |
<0.001 |
|
Uric acid (mg/dL) |
4.5±1.3 |
4.2±1.0 |
0.297 |
|
Insulin (mU/L) |
11.04±5.79 |
9.67±5.15 |
0.472 |
|
Homa-IR |
4.74±2.17 |
2.29±1.3 |
0.001 |
|
WBC ( 109 /L) |
8.31±2.45 |
6.78±2.06 |
0.001 |
Note: eGFR — estimated Glomerular Filtration Rate; AST — Aspartate Transaminase; ALT — Alanine Transaminase; LDL — Low Density Lipoprotein; HDL — High Density Lipoprotein; TyG Index — Triglyceride Glucose Index; HbA1c — Glycated Hemoglobin; CRP — C-Reactive Protein; GGT — Gamma-Glutamyltransferase; Homa-IR — Homeostatic Model Assessment-Insulin Resistance; WBC — White Blood Cell; IMT — Intima Media Thickness.
Table 2: Comparison of conventional echo parameters, diastolic and systolic functions, myocardial velocities, carotid intima-media thickness and epicardial fat thickness in newly diagnosed type 2 diabetes mellitus patients and control groups
|
Newly Diagnosed Type 2 Diabetic (mean+sd) |
Control (mean+sd) |
P |
|
|
EF (%) |
62.97±2.27 |
69.03±1.64 |
<0.001 |
|
LVESD (cm) |
2.52±0.38 |
2.43±0.3 |
0.193 |
|
LVEDD (cm) |
4.52 ±0.41 |
4.5±0.37 |
0.816 |
|
IVS (cm) |
1.1±0.18 |
0.84±0.13 |
<0.001 |
|
PW (cm) |
1.05±0.26 |
0.87 ±0.13 |
<0.001 |
|
Aortic Diameter (cm) |
2.7±0.32 |
2.44±0.31 |
<0.001 |
|
Left Atrium Diameter (cm) |
3.49±0.4 |
3.18±0.41 |
<0.001 |
|
Mitral E (m/sec) |
0.641±0.153 |
0.781±0.103 |
<0.001 |
|
Mitral A (m/sec) |
0.787±0.150 |
0.606±0.088 |
<0.001 |
|
Mitral EDT (ms) |
242.01±66.99 |
193.07±36.19 |
<0.001 |
|
Lateral s’ wave (cm/sec) |
9.82±2.16 |
10.45±2.11 |
0.088 |
|
Lateral e’ wave (cm/sec) |
9.39±2.85 |
12.49±2.84 |
<0.001 |
|
Lateral a’ wave (cm/sec) |
10.68±2.82 |
8.55±1.96 |
<0.001 |
|
Septal s’ wave (cm/sec) |
7.90±1.39 |
8.34±1.29 |
0.063 |
|
Septal e’ wave (cm/sec) |
6.56±1.94 |
9.56±2.05 |
<0.001 |
|
Septal a’ wave (cm/sec) |
9.10±1.94 |
7.43±1.66 |
<0.001 |
|
Tricuspid s’ wave (cm/sec) |
12.68±2.64 |
12.02±1.95 |
0.079 |
|
Tricuspid e’ wave (cm/sec) |
8.82±2.28 |
12.83±2.21 |
<0.001 |
|
Tricuspid a’ wave (cm/sec) |
13.94±2.82 |
9.41±2.11 |
<0.001 |
|
E/A |
0.845±0.274 |
1.311±0.235 |
<0.001 |
|
E/e’ lateral |
07.2±2.1 |
6.4 ±1.6 |
0.026 |
|
E/e’ septal |
10.2±2.6 |
8.4±1.7 |
<0.001 |
|
Carotid IMT (cm) |
0.083±0.022 |
0.053±0.011 |
<0.001 |
|
Epicardial fat thickness (cm) |
0.720±0.198 |
0.382±0.105 |
<0.001 |
Note: EF — Ejection Fraction; LVESD — Left Ventricular End Systolic Diameter; LVEDD — Left Ventricular End Diastolic Diameter; IVS — Interventicular Septum; PW — Posterior Wall; EDT — E Wave Deceleration Time; ET — Ejection Time; IVRT — Isovolumetric Relaxation Time; s’ — Systolic Myocardial Rate; e’ — Early Diastolic Forward Flow Velocity; a’ — Late Diastolic Forward Flow Velocity; IMT — Intima media thickness.
Table 3: Correlation of TyG index measurements with carotid intima-media thickness and epicardial fat thickness measurements
|
Rho |
P |
|
|
Carotid IMT (cm) |
0.343 |
<0.001 |
|
Epicardial fat thickness (cm) |
0.536 |
<0.001 |
|
EF % |
-0.506 |
<0.001 |
|
Waist Circumference (cm) |
0.300 |
0.001 |
|
Hip Circumference (cm) |
0.223 |
<0.018 |
|
BMI (kg/m2) |
0.260 |
0.003 |
|
CRP |
0.346 |
<0.001 |
|
E/A |
-0.468 |
<0.001 |
|
E/e’ septal |
0.278 |
0.001 |
Note: IMT — Intima-media Thickness; EF — Ejection Fraction; CRP — C-reactive protein. Spearman’s correlation test.
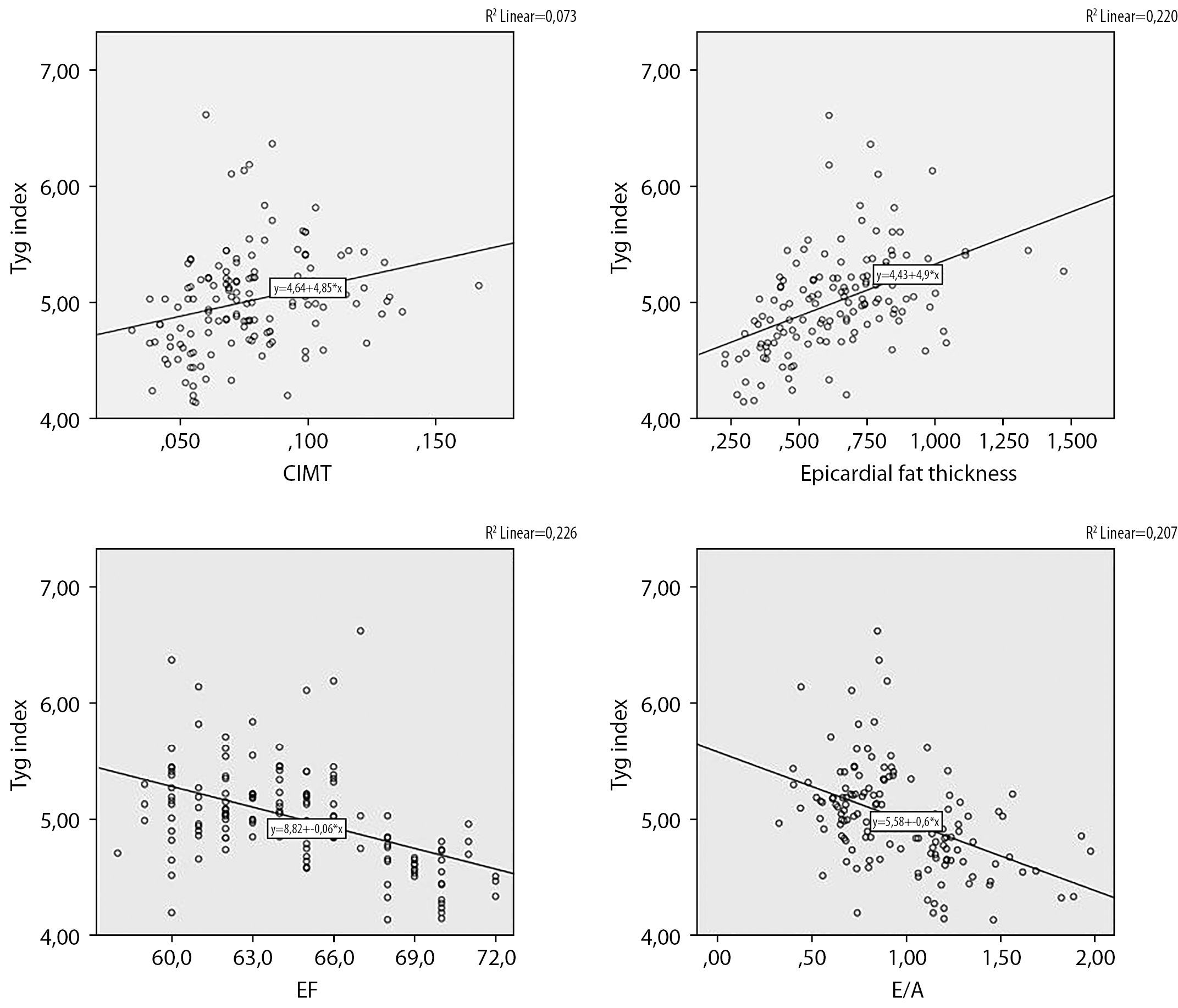
Figure 1: Correlation of Tyg index with carotis intima media thickness, epicardial fat thickness, E/A ratio and ejection fraction.
Table 4: Evaluation of factors affecting Intima-media thickness with regression analysis
|
IMT |
|||
|
β |
%95 CI |
p |
|
|
Age |
0.001 |
0.000–0.001 |
0.001 |
|
TyG Index |
0.010 |
0.001–0.019 |
0.035 |
|
BSA |
0.019 |
-0.002–0.004 |
0.077 |
|
Gender |
-0.005 |
-0.014–0.004 |
0.249 |
|
Smoking |
-0.003 |
-0.011–0.004 |
0.378 |
DISCUSSION
The main result of our study is that, in newly diagnosed type 2 diabetic patients, the TyG index was found to be higher compared to the normal population, and subclinical atherosclerosis was more pronounced than in the normal population even at the stage of newly diagnosed diabetes, and deterioration in myocardial functions was observed at a higher rate than in the normal population. In type 2 diabetic patients, TyG index measurement at the time of diagnosis was associated with subclinical atherosclerosis, deterioration in diastolic functions and deterioration in myocardial functions. In addition, the TyG index may be a predictor of subclinical atherosclerosis and myocardial functions in newly diagnosed type 2 DM individuals.
There is increasing evidence showing that the TyG index, calculated by measuring fasting triglyceride and fasting blood glucose, is correlated with Homa-IR and hyperinsulinemic euglycemic clamp testing [11]. According to a study by Selvi et al. on diabetic patients, a positive correlation was found between TyG index and HbA1c, Homa-IR and BMI [12]. In our study, similar to previous studies, a significant strong positive correlation was found between TyG index and HbA1c and Homa-IR measurement, and a significant moderate positive correlation was found with BMI measurement.
In a meta-analysis that included 5,731,298 patients with no known history of ASCVD to determine the relationship between TyG index and atherosclerotic cardiovascular disease, it was found that higher TyG indices were associated with increased ASCVD, coronary artery disease and stroke. In atherosclerotic cardiovascular disease subgroups examinations, the relationship between the TyG index and the risk of subsequent ASCVD was not affected by age, gender and the presence of diabetes [13]. According to a study published in 2020, higher TyG indices were found to be associated with an increased risk of atherosclerotic cardiovascular diseas and found to be significant in predicting the development of MI and stroke, independent of the presence and number of cardiovascular risk factors [14]. In another study in which 5014 patients were followed-up for 10 years, it was determined that high TyG index was associated with increased development of new atherosclerotic cardiovascular disease, and it was stated that the TyG index could make an additional contribution to the Framingham risk score in predicting the development of ASCVD [15]. In another study conducted with 12,326 participants in South Korea, the TyG index was shown to be an independent marker in predicting coronary artery calcification [16].
In another meta-analysis involving 10,535 patients with coronary artery disease, a relationship was found between the TyG index and the carotid plaque presence. Besides, the relationship was found to be higher in women than in men and also higher in middle-aged and advanced-aged patients compared to advanced-aged ones. The relationship between the TyG index and the presence of carotid plaque was also found to be stronger in the presence of diabetes [17]. In the study conducted by Wu et al., it was determined that the TyG index was positively related with carotid plaque incidence in the general population. It was observed that this relationship continued after patients using antidiabetic, antihypertensive and lipid-lowering drugs were excluded from the study [18]. However, there are also different results. In the study performed by Zhao et al. on 2,830 patients aged 65 and over, they found no correlation between the TyG index and the presence of carotid hypertrophy and carotid plaque [19].
In the study conducted by Irace et al. on 330 patients by calculating Homa-IR values, a relationship was found between TyG index and carotid atherosclerosis, but no relationship was found between Homa-IR measurement and carotid atherosclerosis. The predictive value of the TyG index in detecting carotid atherosclerosis was also confirmed in the second group consisting of 1,432 individuals, in which the Homa-IR calculation was not performed [20]. Our study differs from existing studies in that it was conducted at the time of diagnosis of diabetes and included patients who did not use antihypertensive, antidiabetic and lipid-lowering agents that would affect endothelial functions, and and a statistically significant relationship was found between the TyG index and the carotid IMT thickness. Epicardial fat thickness which may be other echocardiographic indicators of subclinical myocardial involvement, was also evaluated in our study. We found a significant relationship between the TyG index and epicardial fat thickness. According to the literature review, no study was found examining the relationship between the TyG index and epicardial adipose tissue and anterior aortic wall thickness. In our study, a statistically significant relationship was found between TyG index and epicardial adipose tissue thickness. There is also a strong correlation between the TyG index and the measurement of anterior aortic wall thickness. In the regression analysis performed to examine the independent effects of different predictors on carotid IMT and epicardial fat thickness, which can be used as indicators of subclinical atherosclerosis, an increase in TyG index was related with both carotid IMT and epicardial fat thickness. This suggests that the TyG index measurement, which is measured at the time of diagnosis in diabetic patients, can be used to predict subclinical atherosclerosis.
In a study conducted in Taiwan with 823 patients to determine the relationship between TyG index and left ventricular functions, a decrease in left ventricular EF was found with an increase in TyG index [21]. Similarly, in our study, a strong negative correlation was found between TyG index measurements and left ventricular EF in diabetic patients. For this reason, it is thought that TyG index measurements can be used to predict deterioration in systolic function in diabetic patients.
In a study conducted by Parsaee et al. in 2012, tricuspid annular plane systolic excursion and tricuspid E/A ratios were lower in diabetic patients compared to the controls [22]. In a study by Kosmala et al. investigating right ventricular dysfunction in diabetic patients, E was found to be significantly lower, E/A ratio decreased, and isovolumetric relaxation time (IVRT) duration prolonged in the diabetic group [23]. In patients with a normal E/A ratio on Doppler echocardiography, tissue Doppler measurements can be used to detect diabetes-related diastolic dysfunction and increased left ventricular end-diastolic pressure. E/e’ ratio which is an indicator of diastolic dysfunction and increase of left ventricular end-diastolic pressures [24]. In the study of Boyer et al. on diabetic patients, 46% of the patients were diagnosed with diastolic dysfunction by Doppler echocardiography, and this rate was found to be 74% after tissue Doppler measurements [25]. In the study conducted by Zaroufian et al. on 37 people with type 2 diabetes, a decrease in mitral E/A ratio, and an increase in IVRT, mitral EDT and E/e’ ratio were found in diabetic patients. An increased grade 1 diastolic dysfunction was found in the diabetic group compared to the normal population [26]. In our study, patients were evaluated in terms of diastolic dysfunction with Doppler echocardiography and tissue Doppler examination. Similar to other studies, in the newly diagnosed type 2 diabetic group, mitral E decreased, mitral A increased, mitral IVRT and EDT durations were prolonged, E/A ratio decreased, and septal and lateral E/e’ ratios were found to be increased compared to the control group. A study examining the relationship between TyG index and diastolic functions has not been found in the literature. In our study, a moderately significant decrease in mitral E and E/A ratio, a strongly significant increase in mitral A value, and a moderately significant increase in mitral EDT and septal E/e’ ratios were found with increase in TyG index. There was not any correlation between the TyG index and the lateral E/e’ ratio. This suggests that there is an increased presence of diastolic dysfunction in the diabetic group compared to the normal population and that the increase in the TyG index can be used to detect the increased risk of diastolic dysfunction.
Limitations of the study
Our study is primarily a cross-sectional study and individuals who met the study criteria in a certain time period were included. Therefore, the results of the study may not be reflected to the whole population. Newly diagnosed diabetic patients were included in our study, and the duration of diabetes before diagnosis is unknown. In our study, we used carotid IMT thickness and epicardial adipose tissue which are indirect markers of subclinical atherosclerosis. These markers may not fully reflect atherosclerosis in patients. The main parameter affecting the results of our research may be obesity. Since we included patients who met the criteria within a certain period of time in the study, we did not distinguish them as obese or non-obese. However, only 48.5% of the diabetic group was obese. Since patients with no known antihypertensive and lipid-lowering drug use and no history of coronary artery disease are included in the newly diagnosed type 2 diabetic and control patient groups, they do not fully represent the diabetic patient population. However, the direct effect of diabetes on atherosclerosis and myocardial functions was determined due to the inclusion of patients who did not use drugs to affect endothelial functions.
CONCLUSION
According to our study, TyG index is significantly higher in individuals with newly diagnosed type 2 DM compared to the controls. Subclinical atherosclerosis, impairment in systolic and diastolic functions were found in newly diagnosed individuals with type 2 DM. It suggests that there may have been negative changes in systolic and myocardial functions with early atherosclerosis from the early stages of the process leading to type 2 diabetes. TyG index measured at the time of diagnosis in newly diagnosed type 2 diabetic patients is also associated with subclinical atherosclerosis, deterioration in left ventricular systolic and diastolic functions. An increase in the TyG index may predict subclinical atherosclerosis and deterioration in systolic and diastolic myocardial functions. We think that the results of our study should be supported by other studies to be conducted on a larger scale and in different populations.
OTHER INFORMATION
The source of financing. None
Conflicts of interests. None
Participation of authors.
Sena Hekimoglu Ustabas, carried out all the experiment and wrote the manuscript. Esma Güldal Altunoglu, helped supervise the project. Turgut Karabag and Sena Hekimoglu Ustabas assisted in collecting data. All the authors approved the final version of the article before the publication and expressed their consent to be responsible for all aspects of the work, which implies proper investigation and resolving of issues related to the accuracy or integrity of any part of the work.
References
1. International Diabetes Federation. IDF diabetes atlas 2021. IDF Diabetes Atlas. (n.d.). Retrieved March 29, 2022, from https://diabetesatlas.org/atlas/tenth-edition/
2. Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiological Reviews. 2013;93(1):137–188. doi: https://doi.org/10.1152/physrev.00045.2011
3. Haas AV, McDonnell ME. Pathogenesis of cardiovascular disease in diabetes. Endocrinol Metab Clin North Am. 2018;47(1):51–63. doi: https://doi.org/10.1016/j.ecl.2017.10.010
4. Peterson LR, McKenzie CR, Schaffer JE. Diabetic cardiovascular disease: Getting to the heart of the matter. J Cardiovasc Transl Res. 2012;5(4):436–45. doi: https://doi.org/10.1007/s12265-012-9374-7.
5. Poznyak A, Grechko AV, Poggio P, et al. The diabetes mellitus– atherosclerosis connection: The role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. 2020;21(5):1835. doi: https://doi.org/10.3390/ijms21051835
6. American Diabetes Association Professional Practice Committee. 10. cardiovascular disease and risk management: standards of medical care in diabetes — 2022. Diabetes Care. 2022; 45 (Suppl. 1):144-174. doi: https://doi.org/10.2337/dc22-s010
7. Cole JB, Florez J C. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16(7):377–90. doi: https://doi.org/10.1038/s41581-020-0278-5
8. Papatheodorou K, Banach M, Bekiari E, et al. Complications of diabetes 2017. J Diabetes Res. 2018:3086167. doi: https://doi.org/10.1155/2018/3086167
9. Wang L, Cong HL, Zhang JX, et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):80. doi: https://doi.org/10.1155/2018/3086167
10. da Silva A, Caldas APS, Rocha DMUP, et al. Triglycerideglucose index predicts independently type 2 diabetes mellitus risk: A systematic review and meta-analysis of Cohort studies. Prim Care Diabetes. 2020;14(6),584–93. doi: https://doi.org/10.1016/j.pcd.2020.09.001
11. Hameed EK. TyG index a promising biomarker for glycemic control in type 2 diabetes mellitus. Diabetes Metab Syndr. 2019;13(1),560–563. doi: https://doi.org/10.1016/j.dsx.2018.11.030
12. Selvi NMK, Nandhini S, Sakthivadivel V, et al. Association of Triglyceride–Glucose Index (TyG index) with HbA1c and Insulin Resistance in Type 2 Diabetes Mellitus. Maedica (Bucur). 2021;16(3):375-381. doi: https://doi.org/10.26574/maedica.2021.16.3.375
13. Ding X, Wang X, Wu J, et al. Triglyceride–glucose index and the incidence of atherosclerotic cardiovascular diseases: A metaanalysis of Cohort studies. Cardiovasc Diabetol. 2021;20(1):76. doi: https://doi.org/10.1186/s12933-021-01268-9
14. Hong S, Han K, Park CY. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: A population-based study. BMC Med. 2020;18(1):361. doi: https://doi.org/10.1186/s12916-020-01824-2
15. Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, et al. The TYG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2),189–197. doi: https://doi.org/10.1111/eci.12583
16. Won KB, Park EJ, Han D, et al. Triglyceride glucose index is an independent predictor for the progression of coronary artery calcification in the absence of heavy coronary artery calcification at baseline. Cardiovasc Diabetol. 2020;19(1): 34. doi: https://doi.org/10.1186/s12933-020-01008-5
17. Li Z, He Y, Wang S, et al. Association between triglyceride glucose index and carotid artery plaque in different glucose metabolic states in patients with coronary heart disease: A RCSCD-TCM study in China. Cardiovascr Diabetol. 2022;21(1):38. doi: https://doi.org/10.1186/s12933-022-01470-3
18. Wu Z, Wang J, Li Z, et al. Triglyceride glucose index and carotid atherosclerosis incidence in the Chinese population: A prospective cohort study. Nutr Metab Cardiovasc Dis. 2021;31(7), 2042–50. doi: https://doi.org/10.1016/j.numecd.2021.03.027
19. Zhao S, Yu S, Chi C, et al. Association between macroand microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: The Northern Shanghai Study. Cardiovasc Diabetol. 2019;18(1):95. doi: https://doi.org/10.1186/s12933-019-0898-x
20. Irace C, Carallo C, Scavelli FB, et al. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and Triglyceride Glucose Index. Int J Clin Pract. 2013;67(7),665–672. doi: https://doi.org/10.1111/ijcp.12124
21. Chiu TH, Tsai HJ, Chiou HYC, et al. A high triglycerideglucose index is associated with left ventricular dysfunction and atherosclerosis. Int J Med Sci. 2021;18(4),1051–1057. doi: https://doi.org/10.7150/ijms.53920
22. Parsaee M, Bahmanziari P, Ardeshiri M, et al. Obvious or Subclinical Right Ventricular Dysfunction in Diabetes Mellitus (Type II): An Echocardiographic Tissue Deformation Study. J Tehran Heart Cent. 2012;7(4),177–181
23. Kosmala W, Colonna P, Przewlocka-Kosmala M, et al. Right ventricular dysfunction in asymptomatic diabetic patients. Diabetes Care. 2004;27(11),2736–2738. doi: https://doi.org/10.2337/diacare.27.11.2736
24. Murtaza G, Virk HU, Khalid M, et al. Diabetic cardiomyopathy - a comprehensive updated review. Prog Cardiovasc Dis. 2019;62(4), 315–326. doi: https://doi.org/10.1016/j.pcad.2019.03.003
25. Boyer J K, Thanigaraj S, Schechtman KB, et al. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. The American Journal of Cardiology. 2004; 93(7), 870–875. doi: https://doi.org/10.1016/j.amjcard.2003.12.026
26. Zoroufian A, Razmi T, Taghavi-Shavazi M, Lotfi-Tokaldany M, Jalali A. Evaluation of subclinical left ventricular dysfunction in diabetic patients: Longitudinal strain velocities and left ventricular dyssynchrony by twodimensional speckle tracking echocardiography study. Echocardiography. 2013;31(4),456–463. doi: https://doi.org/10.1111/echo.1238
About the Authors
S. H. UstabasTurkey
Sena Hekimoglu Ustabas, Research Assistant
Istanbul
E. G. Altunoglu
Turkey
Esma Guldal Altunoglu, Professor Dr.
Istanbul
T. Karabag
Turkey
Turgut Karabag, Professor Dr.
Istanbul Egitim Arastırma Hastanesi, Kardiyoloji Bölümü, Kat:6 Fatih
Supplementary files
|
|
1. Figure 1: Correlation of Tyg index with carotis intima media thickness, epicardial fat thickness, E/A ratio and ejection fraction. | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(347KB)
|
Indexing metadata ▾ | |
Review
For citations:
Ustabas S.H., Altunoglu E.G., Karabag T. Triglyceride glucose index is associated with subclinical atherosclerosis and subclinical myocardial dysfunction in patients with newly diagnosed type 2 diabetes mellitus. Diabetes mellitus. 2024;27(3):224-232. https://doi.org/10.14341/DM13073

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0).















































