Перейти к:
Спектр эффектов ингибиторов дипептидилпептидазы-4: внутри и за пределами гликемического контроля (часть 1)
https://doi.org/10.14341/DM13342
Аннотация
Современные акценты контроля сахарного диабета 2 типа (СД2) сместились с HbA1c на вариабельность гликемии (ВГ) вследствие ее ключевого значения в ускоренном развитии диабетических осложнений, помимо хронической гипергликемии. Центральным звеном ранней стадии дисгликемии служит дисфункция β-клеток с последующей потерей их массы при важной роли гиперглюкагонемии на всех этапах диабетического континуума. Решающее значение для поддержания гомеостаза глюкозы имеет скоординированная работа α и β-клеток с помощью двух эндогенных инкретинов: глюкагоноподобного пептида-1 (ГПП-1) и глюкозозависимого инсулинотропного полипептида (ГИП). Способность ингибиторов дипептидилпептидазы-4 (иДПП-4) за счет сохранения биоактивных ГПП-1 и ГИП интактными не только поддерживать массу β-клеток и способствовать высвобождению инсулина, а также одновременно корректировать секрецию глюкагона из α-клеток, предотвращая гипогликемии, привлекает к препаратам особое внимание.
Рассматривается место иДПП-4 среди различных фармакологических вариантов лечения СД2: уточняются детали гликемического контроля и роль в снижении ВГ с безопасностью в отношении риска сердечно-сосудистых заболеваний (ССЗ). Представлены новые данные о механизмах действия дипептидазы-4, которая, как новый адипокин с системной активностью и клеточной специфичностью в регуляции не только метаболического гомеостаза, но и воспалительных процессов, может представлять собой ключевое звено между центральным ожирением, инсулинорезистентностью (ИР) и атеросклерозом. Соответственно, патофизиологическая связь СД2 и ССЗ через ИР и низкоуровневое воспаление определила смещение целей терапии с контроля уровня глюкозы крови на общее управление факторами риска, в котором уточняется роль и место иДПП-4.
Ключевые слова
Для цитирования:
Руяткина Л.А., Руяткин Д.С. Спектр эффектов ингибиторов дипептидилпептидазы-4: внутри и за пределами гликемического контроля (часть 1). Сахарный диабет. 2025;28(4):404-412. https://doi.org/10.14341/DM13342
For citation:
Ruyatkina L.A., Ruyatkin D.S. Spectrum of effects of dipeptidyl peptidase-4 inhibitors: within and beyond glycemic control (part 1). Diabetes mellitus. 2025;28(4):404-412. (In Russ.) https://doi.org/10.14341/DM13342
НОВЫЕ ПОДХОДЫ К КОНТРОЛЮ САХАРНОГО ДИАБЕТА 2 ТИПА
Глобальная распространенность сахарного диабета 2 типа (СД2) привела к широкому распространению мультисистемных повреждений, особенно сердечно-сосудистых заболеваний (ССЗ) и почечных дисфункций в рамках кардиоренального континуума, что повышает заболеваемость и смертность [1]. Ключевую роль в патогенезе сосудистых осложнений диабета играет низкоуровневое воспаление (НУВ) в тесной связи с инсулинорезистентностью (ИР) и окислительным стрессом (ОС) [2][3]. Метаболические нарушения вследствие ИР и гипергликемии, усиливая провоспалительный фенотип и ОС, могут привести к повреждению сосудов и миокарда [4][5].
В современных парадигмах лечения диабета основное внимание сместилось с гликемического контроля на общее управление факторами риска с корректировкой индивидуальных целевых показателей гликемии [6]. Введение непрерывного мониторинга глюкозы открыло новую эру в клинической практике, сместив характеристику гликемического контроля с HbA1c на новые показатели — вариабельность гликемии (ВГ), тесно связанную с риском гипогликемических событий [7]. Обосновано мнение о ключевой роли ВГ, помимо хронической гипергликемии, в ускоренном развитии диабетических осложнений [8][9] независимо от HbA1c [10], поскольку более жесткий контроль HbA1c сам по себе недостаточен для предотвращения сердечно-сосудистых событий, вызванных длительной высокой ВГ [11].
Метаанализ 71 исследования показал, что повышенная уже в ситуации предиабета ВГ потенциально связана с дисфункцией β-клеток и развитием коронарного атеросклероза и может предсказывать ССЗ и СД2 [12]. Более высокая ВГ тесно связана с уязвимостью коронарных бляшек [7][13] и прогрессированием атеросклероза сонной артерии у пациентов с СД2 [14]. Доказана способность индексов ВГ, в том числе TIR (Time-In-Range, время в целевом диапазоне), предсказывать клинические осложнения диабета [14][15][16].
Воздействие ВГ на органы-мишени может быть реализовано через ОС, гликирование, хроническое НУВ, эндотелиальную дисфункцию, активацию тромбоцитов, нарушение ангиогенеза и почечный фиброз [17]. Это влияние ВГ на механизмы реализации сердечно-сосудистых осложнений в тесной связи с риском гипогликемий меняет подходы к контролю диабета, предполагая влияние новых сахароснижающих препаратов (инкретиновых или ингибиторов натрий-глюкозного транспортера-2 (иНГЛТ-2) на снижение степени ВГ [18]. Подобный подход обосновывает изменение терминологии в тесной связи с актуальной стратегией: «антигипергликемические» препараты вместо «сахароснижающих». Патофизиологическая связь СД2 и ССЗ, позволяя получать препараты с благоприятным воздействием на оба этих состояния [19], определяет термин «антидиабетические» препараты по отношению к ряду новых лекарственных средств, изначально предназначенных для контроля гипергликемии.
НОВЫЕ АНТИДИАБЕТИЧЕСКИЕ ПРЕПАРАТЫ: ВОПРОСЫ К СЕРДЕЧНО-СОСУДИСТОЙ БЕЗОПАСНОСТИ
Результаты многочисленных плацебо-контролируемых рандомизированных клинических исследований (РКИ) сердечно-сосудистых исходов (CVOT) при СД2, продемонстрировав способность новейших классов антидиабетических препаратов, иНГЛТ-2 и агонистов рецепторов глюкагон-подобного пептида-1 (арГПП-1) снижать основные неблагоприятные сердечно-сосудистые события, позволили им занять видное место в алгоритмах лечения СД2 [20] и способствовали быстрому развитию кардиоэндокринологии [21][22]. К новым антигипергликемическим препаратам также относят ингибиторы дипептидилпептидазы-4 (иДПП-4), показавшие эффективность в сохранении сердечной и почечной функции, как у больных СД2, так и у лиц без него [1].
Синергические механизмы влияния трех групп новых антигипергликемических препаратов на сердечно-сосудистый прогноз (рис. 1) включают, кроме классических факторов патогенеза ССЗ (ИР, гипергликемию и гиперлипидемию), воспаление и ОС, способствующих митохондриальной дисфункции с последующим развитием фибротических процессов в миокарде [22]. Caturano A. и соавт. (2024), анализируя ключевую роль оси «инсулин-сердце» в патофизиологии ССЗ при инсулинорезистентных состояниях, включая диабет, отмечают, что метформин, иНГЛТ-2, арГПП-1 и иДПП-4 показали эффективность в снижении сердечно-сосудистого риска за счет устранения метаболического дисбаланса, уменьшения воспаления и улучшения эндотелиальной функции [23].
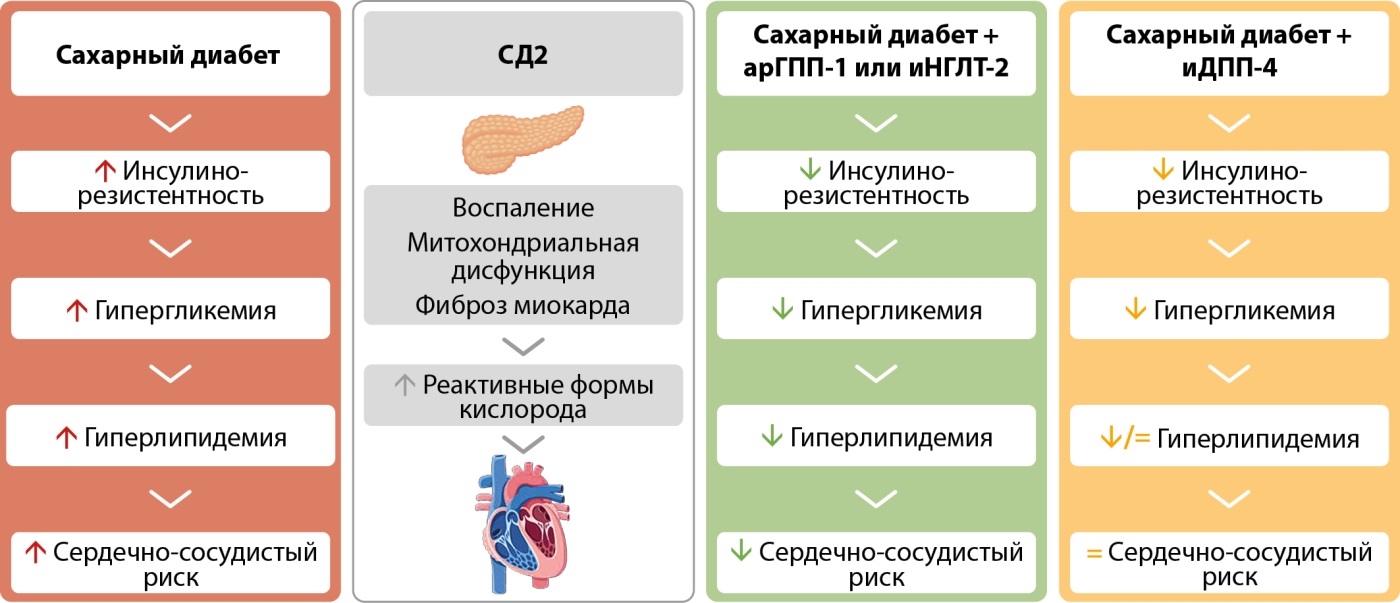
Рисунок 1. Современные фармакологические варианты лечения сахарного диабета 2 типа и их эффективность в отношении сердечно-сосудистого риска. Адаптировано по Andreadi A. и соавт. (2023) [22].
Примечание: á: увеличение, â: уменьшение, арГПП-1 — агонисты рецепторов глюкагоноподобного пептида 1; иНГЛТ-2 — ингибиторы натрий-глюкозного котранспортера 2-го типа.
Три отдельных клинических нозологии: ишемическая болезнь сердца, кардиальная автономная нейропатия и диабетическая кардиомиопатия (ДКМП) — в совокупности образуют диабетическую болезнь сердца [24]. При этом ДКМП — самая важная причина диастолической и систолической хронической сердечной недостаточности (ХСН), связана со значительно более высоким риском заболеваемости и смертности, а существующая терапия ДКМП в основном имеет симптоматический характер [25]. Уточняя различия в механизмах кардиопротекторного влияния иНГЛТ-2, арГПП-1 и иДПП-4 (рис. 2) с особым вниманием к воспалению, ОС и фиброзу [26], делается особый акцент на высоковоспалительный режим апоптоза, пироптоз, участвующий в патогенезе атеросклероза, ишемически-реперфузионного повреждения и ДКМП [25].
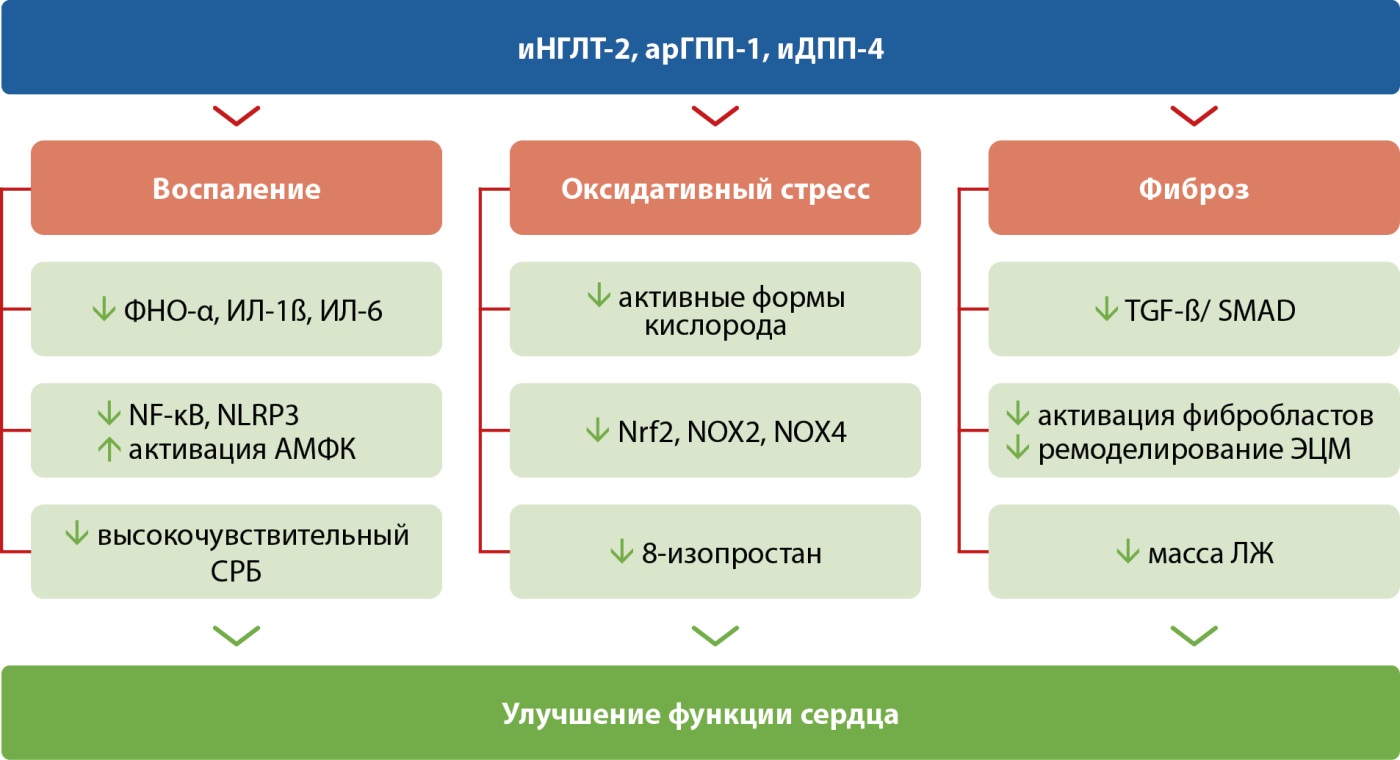
Рисунок 2. Влияние ингибиторов натрий-глюкозного котранспортера 2-го типа, агонистов рецепторов глюкагоноподобного пептида-1 и ингибиторов дипептидилпептидазы-4 на воспаление, окислительный стресс и фиброз. Адаптировано по Balogh D.B. и соавт. (2023) [26].
Примечание: АМФК — аденозинмонофосфат-активируемая протеинкиназа; ИЛ-1ß — интерлейкин 1ß; ИЛ-6 — интерлейкин 6; ЛЖ — левый желудочек; СРБ — С-реактивный белок; ФНО-α — фактор некроза опухоли α; ЭЦМ — экстрацеллюлярный матрикс; NF-ĸB (nuclear factor-ĸB (англ.)) — ядерный фактор-ĸB; NLRP3 (Nod-like receptor (NLR) family pyrin domain-containing 3 (англ.)) — рецептор семейства Nod-подобных рецепторов, содержащий пириновый домен 3; NOX (NADPH oxidase (англ.)) — НАДФН-оксидаза (никотинамидадениндинуклеотидфосфат восстановленный); Nrf2 (nuclear factor erythroid 2-related factor 2 (англ.)) — ядерный фактор эритроидного происхождения 2; SMAD — белок, кодируемый геном SMAD; TGF-ß (Transforming growth factor-ß (англ.)) — трансформирующий фактор роста ß.
Ингибиторы ДПП-4 наряду с метформином, иНГЛТ-2 и тиазолидиндионами улучшают ДКМП [25]. Обосновывается применение иДПП-4 для профилактики гипертрофии сердца и его диастолической дисфункции путем ингибирования ОС и фиброза [27]. При этом иДПП-4 (глиптины) демонстрируют нейтральное влияние на сердечно-сосудистую смертность, госпитализацию с ХСН, риск инфаркта миокарда, неотложной коронарной реваскуляризации, инсульта [1][19][23][28]. Важно отметить, что эти клинические исследования были разработаны по принципу не меньшей эффективности, подтверждая сердечно-сосудистую безопасность этих препаратов, а не обязательно их наблюдаемые преимущества [29].
Несмотря на инкретиновый эффект, потенциальные кардиопротекторные преимущества, обеспечиваемые арГПП-1, не были воспроизведены в CVOT, оценивающих иДПП-4 [21]. Однако в post-hoc-анализе EXAMINE у пациентов с расчетной скоростью клубочковой фильтрации (рСКФ) ≥60 мл/мин 1,73 м² в группе алоглиптина выявлено снижение риска MACE (серьезных неблагоприятных сердечно-сосудистых событий) на 19% (р=0,014), сердечно-сосудистой смертности на 39% (р=0,013) и нефатального инфаркта миокарда на 14% (р=0,013) [30]. Эти данные коррелируют с результатами общенационального когортного исследования Тайваня, где было обнаружено, что терапия иДПП-4 ассоциируется со снижением риска MACE в когорте без хронической болезни почек [31]. Аналогично в ретроспективной когорте США, включившей 445 701 пациента, впервые прошедшего лечение по поводу СД2, глиптины продемонстрировали снижение риска MACE на 13% по сравнению с сульфонилмочевиной (СМ) при аналогичном с метформином риске MACE [32]. Отметим, что схожие результаты получены в трех различных исследованиях на больших этнически разнообразных группах пациентов среднего возраста без сердечно-сосудистых или почечных заболеваний, начинающих пероральную антигипергликемическую терапию, что по оценкам составляет 69% вновь диагностированных пациентов [33].
ИНГИБИТОРЫ ДПП-4: ДЕТАЛИ ГЛИКЕМИЧЕСКОГО КОНТРОЛЯ
Фермент ДПП-4, сериновая протеаза, сам по себе не оказывает сахароснижающего действия. Особое внимание привлекает инкретиновый эффект иДПП-4. Гомеостаз глюкозы тонко регулируется путем сбалансированного действия инсулина и глюкагона посредством механизмов обратной связи и перекрестных помех между различными органами, включая поджелудочную железу, печень, скелетные мышцы и жировую ткань [34]. По мере прогрессирования дисгликемии, повышения ИР и снижения функции β-клеток, постепенно увеличивается концентрация глюкагона, что объяснили наличием рецепторов к инсулину у α-клеток поджелудочной железы, в результате чего ИР способствует нарушению подавления глюкагона при СД2 [34]. Сформулирована концепция, согласно которой токсичность глюкозы может проявляться сначала как дисфункция α-клеток, предваряя истощение секреции инсулина [35].
Итак, молекулярные основы СД2 включают ИР, недостаточное действие инсулина и аберрантную секрецию глюкагона, в совокупности нарушая тесную координацию метаболических процессов, способствуя гипергликемии и дислипидемии [36]. Поскольку гипергликемия при диабете возникает только при нарушении функции β-клеток [37], в настоящее время предпочтение отдается β-центричному лечению. Дисфункция β-клеток является ключевым звеном ранней стадии дисгликемии, на более поздних стадиях преобладает общая потеря массы β-клеток [38] при важной роли гиперглюкагонемии на всех этапах диабетического континуума [35][39][40][41]. Способность иДПП-4 сохранять массу и функции β-клеток с помощью двух эндогенных инкретинов: ГПП-1 и глюкозозависимого инсулинотропного полипептида (ГИП) — имеет решающее значение для поддержания гомеостаза глюкозы, привлекая к глиптинам особое внимание [42]. Их двойное действие на островковые α- и β-клетки за счет сохранения биоактивных ГПП-1 и ГИП интактными способствует их ситуационной функциональной активности в зависимости от уровней гликемии. Так, в постпрандиальном периоде повышается секреция инсулина и снижается — глюкагона, натощак наоборот: снижается секреция инсулина и повышается — глюкагона, что предотвращает риск гипогликемии, характеризуя глюкозозависимый антигипергликемический эффект [43][44], в отличие от дозозависимого сахароснижающего.
Отражение в гайдлайнах Америкаснкой диабетической ассоциации (ADA) и Европейской ассоциации по изучению сахарного диабета (EASD) низкого риска гипогликемий у всех трех групп новых антигипергликемических препаратов: арГПП-1, иНГЛТ-2 и иДПП-4 — подчеркивает их сильную сторону в терапии СД2 [26]. Именно меньшая опасность гипогликемии пропорционально величине снижения HbA1c является ключевым фактором в снижении риска MACE независимо от используемых препаратов [45]. На основе серьезного анализа публикаций в Pubmed риск гипогликемий, сравнимый с плацебо, выявлен у иДПП-4 (1,0) в отличие от длительно действующих аналогов ГПП-1 (1,8) и кратно более высокого у СМ (3,6–10,2) [46], что также отражает потенциальные преимущества для сердечно-сосудистой системы [47].
Ингибиторы ДПП-4 являются многообещающей альтернативой для снижения ВГ у пациентов с СД2 [48] по сравнению с СМ и иНГЛТ-2 [49], высокой дозой метформина и его комбинации с многократной ежедневной инсулинотерапией (по данным проспективного РКИ) [50]. Этот эффект иДПП-4 особенно значим по сравнению с СМ и ингибиторами НГЛТ-2 у пациентов с длительностью СД2≤5 лет по сравнению с пациентами с длительностью заболевания >5 лет [49]. Интерес к иДПП-4 повысился с учетом доказательств их наиболее благоприятного профиля переносимости и безопасности, в том числе снижению MACE по сравнению с таковой у препаратов СМ с аналогичной эффективностью [51][52], отсутствием увеличения веса и необходимости повышения дозы [20], а также эффективностью в снижении уровня циркулирующего постпрандиального глюкагона [39].
Нуждается в анализе более высокий риск гипогликемий у арГПП-1 при их более высокой сахароснижающей эффективности: HbA1c -1,77% у семаглутида в дозе 1 мг в сравнении с -0,66% у иДПП-4 [46]. Обусловлено ли это только разными уровнями ГПП-1 в крови при лечении арГПП-1 (фармакологические) или иДПП-4 (физиологические)? Механистическая интерпретация эффектов ингибирования фермента: увеличение периода полураспада ГПП-1 до нормальных уровней в плазме крови, что помогает восстановить функцию β-клеток, улучшить секрецию инсулина и сдержать секрецию глюкагона α-клетками, не объясняет этого факта [53], определяя интерес к спектру возможностей иДПП-4 и различиям с арГПП-1.
Отметим, что в антигипергликемическом действии иДПП-4 произошла переоценка роли ГИП [20] не только вследствие его способности улучшать метаболизм глюкозы и липидов, особенно в сочетании с механизмом ГПП-1 [54][55]. Два инкретина имеют как общие, так и различные функции. По образному выражению Nauck M.A. и Meier J.J. (2019) «ГИП и ГПП-1: сводные братья и сестры, а не монозиготные близнецы в семье инкретинов». Так, ГИП обладает более мощным инсулинотропным действием и отвечает за 44% от общего количества инсулиновых ответов, а вклад ГПП-1 составляет 22% [56]. В отличие от ГПП-1, ГИП не влияет на концентрацию глюкагона во время гипергликемии, однако он увеличивает его уровни натощак и в условиях гипогликемии, что может способствовать снижению риска тяжелой гипогликемии у пациентов с СД2 [55][57][58]. ГИП также оказывает пролиферативное действие на бета-клетки [59]. Интересно, что пептид YY (PYY) также является субстратом ДПП-4, а ингибиторы фермента снижают превращение PYY в его анорексигенные метаболиты — эффект, который может противодействовать эффекту потери веса от повышенного ГПП-1 и поддерживать нейтральность веса у иДПП-4 [60].
АНТИДИАБЕТИЧЕСКИЕ ЭФФЕКТЫ ИДПП-4: ВЗГЛЯД ЗА ПРЕДЕЛЫ HbA1c
Пробелы в знаниях о функции ДПП-4 и расхождение сердечно-сосудистых исходов, наблюдаемых в доклинических и крупномасштабных РКИ с иДПП-4, требуют дополнительных исследований [61]. Накапливающиеся данные показывают потенциальные преимущества иДПП-4 при различных ССЗ, включая артериальную гипертонию, кальцинированный аортальный стеноз, коронарный атеросклероз и ХСН. В дополнение к улучшению глюкозного гомеостаза, иДПП-4 участвуют в контроле сердечно-сосудистых факторов риска, в том числе постпрандиального липидного метаболизма [36]. Также они напрямую регулируют возникновение и прогрессирование ССЗ посредством различных механизмов [62].
Недавние исследования расширили понимание механизмов действия иДПП-4. ДПП-4, также известная как поверхностный антиген Т-клеток CD26, представляет собой трансмембранную сериновую протеазу, экспрессируемую на поверхности различных клеток, существует как в мембран-связанной, так и в растворимой формах. Растворимая ДПП-4 ферментативно активна и участвует в различных физиологических и патологических процессах: от гомеостаза глюкозы, иммунорегуляции, воспаления до онкогенеза [44]. По сути, она идентифицирована как новый адипокин с системной активностью в регуляции не только метаболического гомеостаза, но и воспалительных процессов [63].
Гипотетическая траектория НУВ отклоняется от здорового состояния за несколько лет до развития СД2 при наличии предиабета или других метаболических нарушений [3], согласуясь с данными NHANES о связи дисгликемии с индексом системного иммунного воспаления: его повышенные уровни связаны с повышенным риском СД2, а у лиц с СД — с повышенным риском смертности от всех причин и ССЗ [64][65]. Поскольку активность НУВ, ключевого фактора СД2 и связанных с ним ССЗ остается высокой даже после лечения (некоторые долгосрочные воспалительные пути не затрагиваются обычными методами лечения), возникают новые требования к препаратам, способным через этот путь обратить вспять раннюю стадию СД2. Инновационные исследования разрабатывают терапевтические стратегии для предотвращения патогенных механизмов и защиты бета-клеток от воздействия воспаления и/или хронической гипергликемии [66].
Уже не вызывает сомнений, что иДПП-4, помимо влияния на β-клетку, участвуют в коррекции ИР и модуляции воспаления, то есть ведущих факторах патогенеза СД2 [23]. Лечение иДПП-4 улучшает гликемический контроль как за счет увеличения секреции инсулина из островковых клеток, снижения уровня HbA1c, так и уменьшения размера адипоцитов и подавления воспаления [29][36]. Висцеральная жировая ткань является основным источником провоспалительных адипокинов [23]. ДПП-4 расщепляет и инактивирует несколько хемокинов и цитокинов, оказывая значительное влияние на воспаление и [67] и может представлять собой ключевое звено между центральным ожирением, ИР и атеросклерозом (рис. 3) [61]. При этом важен антиоксидантный потенциал иДПП-4 в зависимом и независимом от ГПП-1 режиме при окислительном стрессе [53].
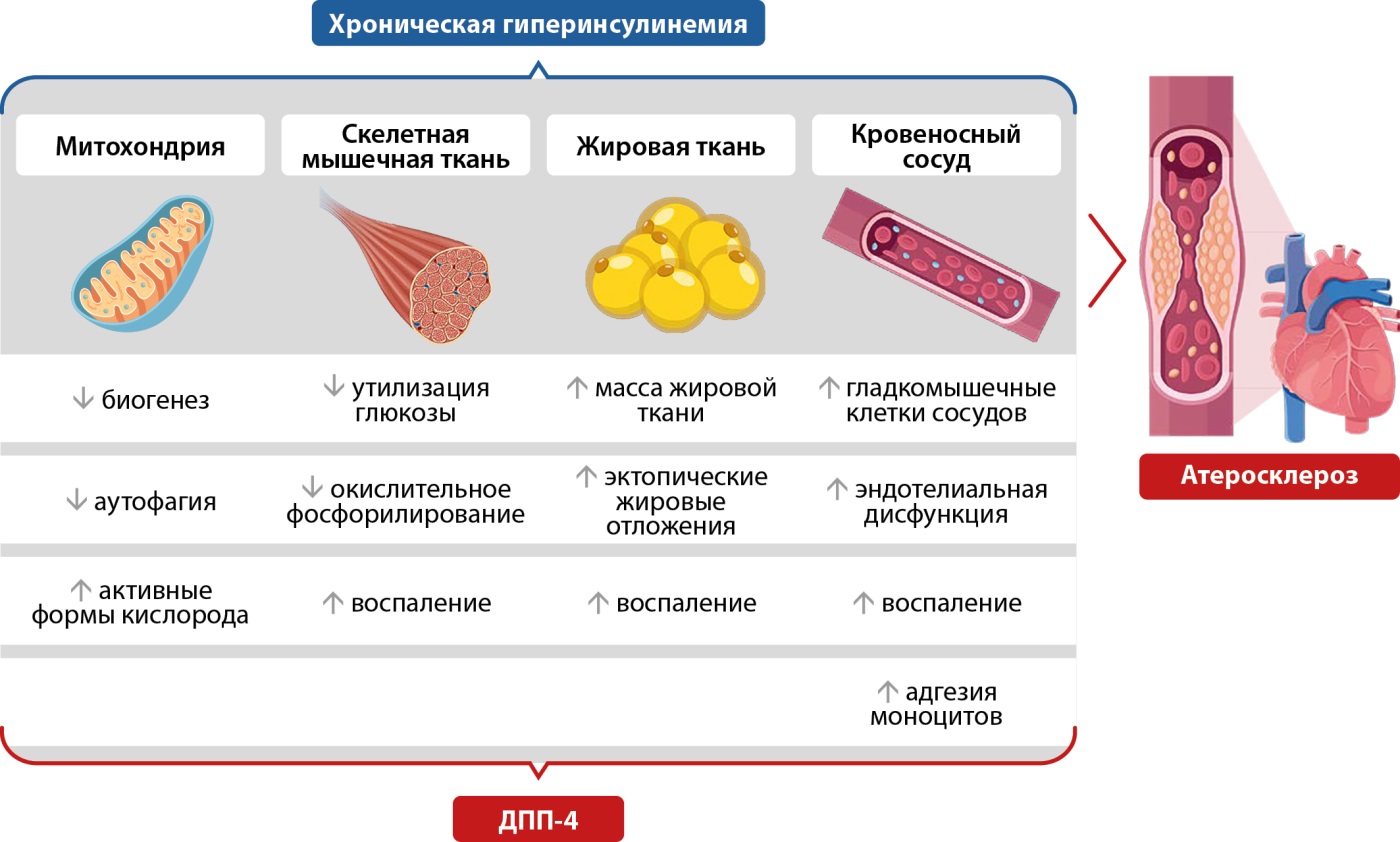
Рисунок 3. Возможные механизмы, лежащие в основе дипептидилпептидазы-4-опосредованного атеросклероза в состоянии инсулинорезистентности. Адаптировано по Love K.M. и Liu Z. (2021) [61].
Как белок-командир, ДПП-4 обладает множественными функциями, ферментативными и неферментативными, в различных типах клеток. Среди новых ее функций — содействие фиброзу и проникновению вируса. Вследствие участия в фибротическом ответе и иммунорегуляции все больше исследований фокусируются на потенциальной роли ДПП-4 при воспалительных расстройствах и регуляции иммунной функции [68]. Неспособность разрешить воспаление, приводящее к хронической субклинической активации иммунной системы, может влиять на развитие метаболической дисрегуляции. Таким образом, ДПП-4 проявляет разнообразный спектр эффектов (рис. 4), которые могут влиять на прогрессирование метаболического заболевания [69], в том числе через сигнальный путь инсулина, способствуя ИР и вовлечению в воспалительные процессы, такие как ожирение и СД2 [70]. Подчеркивается потенциал ДПП-4 в качестве мишени для лечения различных иммунных дисфункций [71].
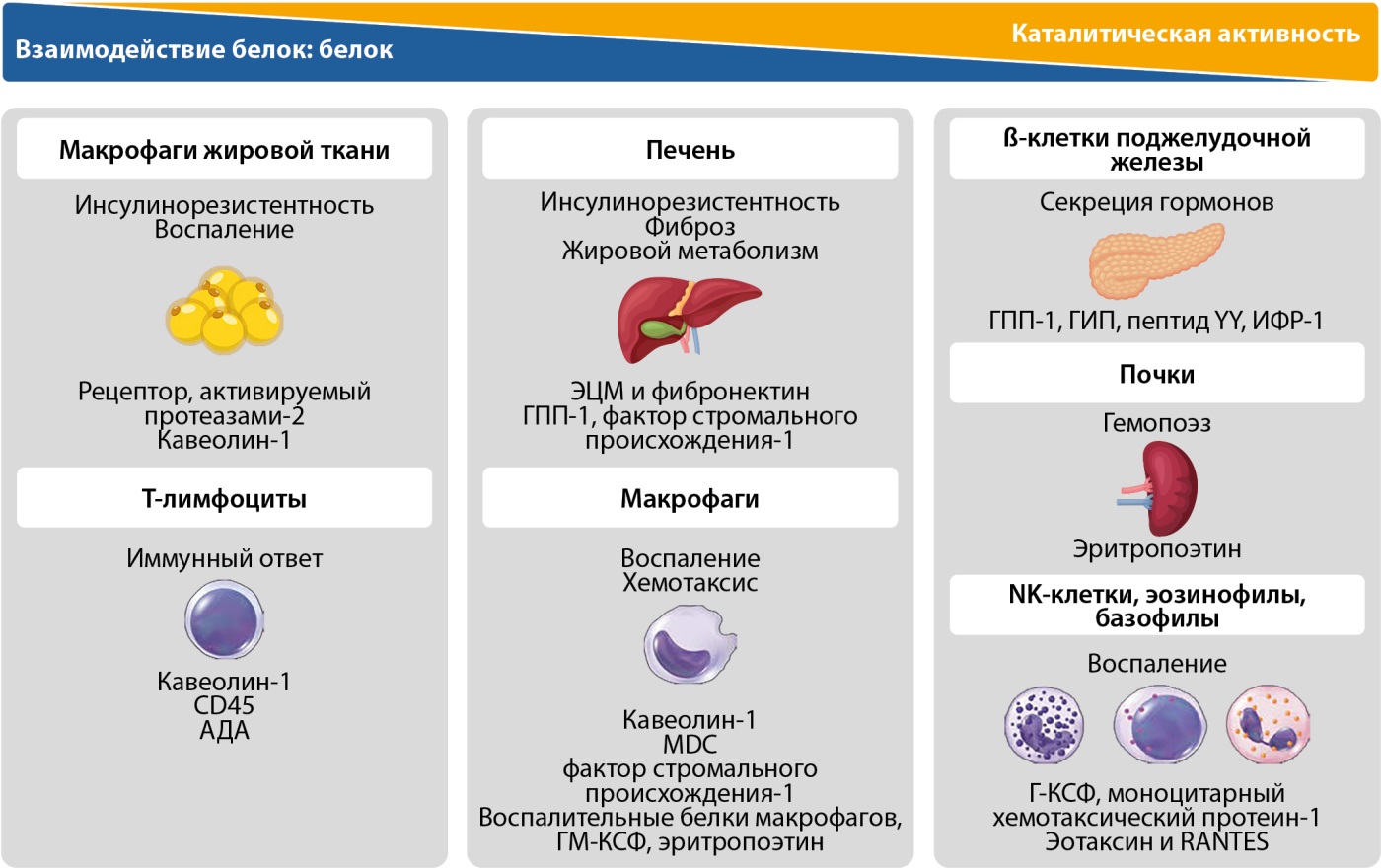
Рисунок 4. Метаболические эффекты и клеточная специфичность дипептидилпептидазы-4 в отношении толерантности к глюкозе и воспаления. Адаптировано по Trzaskalski N.A. и соавт. (2020) [69].
Примечание: АДА — аденозиндезаминаза; Г-КСФ — гранулоцитарный колониестимулирующий фактор; ГМ-КСФ — гранулоцитарно-макрофагальный колониестимулирующий фактор; ИФР-1 — инсулиноподобный фактор роста-1; пептид YY — пептид тирозин-тирозин; ЭЦМ — экстрацеллюлярный матрикс; CD45 (cluster of differentiation 45 (англ.)) — дифференцировочный антиген лейкоцитов; MDC (macrophage-derived cytokine (англ.)) — макрофагальный хемокин; RANTES (regulated on activation, normal T-cell expressed and secreted (англ.)) — рекомбинантный, регулируемый при активации, экспрессируемый и секретируемый нормальными Т-клетками хемокин.
ЗАКЛЮЧЕНИЕ
Мультисистемные повреждения при СД2 в рамках кардио-гепаторенального континуума и патофизиологическая связь СД2 и ССЗ через ИР, НУВ и оксидативный стресс послужили основой концепции кардиодиабетологии, сместив цели терапии с контроля глюкозного гомеостаза на общее управление факторами риска. Значение ВГ в ускоренном развитии диабетических осложнений, наряду с недостаточностью более жесткого контроля HbA1c для предотвращения сердечно-сосудистых событий, вызванных длительной высокой ВГ, выдвинуло особые требования к антигипергликемическим препаратам: снижение ВГ, тесно связанной с риском гипогликемий.
Тесная связь ВГ с дисбалансом функционального состояния β-клеток, ключевого звена ранней стадии дисгликемии, и α-клеток с важной ролью гиперглюкагонемии на всех этапах диабетического континуума, привлекает внимание к разноплановым возможностям иДПП-4. Эти препараты, сохраняя интактными два инкретина, ГПП-1 и ГИП, помимо скоординированного влияния на β-клетку и α-клетку со снижением риска гипогликемий, участвуют в коррекции ИР, модуляции окислительного стресса и воспаления — ведущих факторов патогенеза СД2, связывающие его с формированием атеросклероза. Максимальный эффект иДПП-4 в отношении снижения ВГ отмечен при длительности СД2≤5 лет при сравнении с препаратами СМ и глифлозинами, а также риска МАСЕ по сравнению с таковой у препаратов СМ с аналогичной эффективностью.
ДОПОЛНИТЕЛЬНАЯ ИНФОРМАЦИЯ
Источник финансирования. Статья подготовлена при поддержке группы компаний «НИЖФАРМ».
Конфликт интересов. Авторы декларируют отсутствие конфликта интересов.
Вклад авторов. Руяткина Л.А. — концепция, анализ литературных данных, написание статьи; Руяткин Д.С. — обсуждение концепции, поиск и анализ литературных данных, написание статьи. Все авторы одобрили финальную версию статьи перед публикацией, выразили согласие нести ответственность за все аспекты работы, подразумевающую надлежащее изучение и решение вопросов, связанных с точностью или добросовестностью любой части работы.
Список литературы
1. Fu WJ, Huo JL, Mao ZH, et al. Emerging role of antidiabetic drugs in cardiorenal protection. Front Pharmacol. 2024;15:1349069. doi: https://doi.org/10.3389/fphar.2024.1349069
2. Zhao L, Hu H, Zhang L, et al. Inflammation in diabetes complications: molecular mechanisms and therapeutic interventions. MedComm (2020). 2024;5(4):e516. doi: https://doi.org/10.1002/mco2.516
3. Pellegrini V, La Grotta R, Carreras F, et al. Inflammatory Trajectory of Type 2 Diabetes: Novel Opportunities for Early and Late Treatment. Cells. 2024;13(19):1662. doi: https://doi.org/10.3390/cells13191662
4. Yun JS, Ko SH. Current trends in epidemiology of cardiovascular disease and cardiovascular risk management in type 2 diabetes. Metabolism. 2021;123:154838. doi: https://doi.org/10.1016/j.metabol.2021.154838
5. Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020;17(12):761-772. doi: https://doi.org/10.1038/s41569-020-0406-8
6. Дедов И.И., Шестакова М.В., Майоров А.Ю., и др. Алгоритмы специализированной медицинской помощи больным сахарным диабетом / Под ред. И.И. Дедова, М.В. Шестаковой, А.Ю. Майорова. 11-й выпуск // Сахарный диабет. — 2023. — Т. 26. — №2S. — С. 1-157. doi: https://doi.org/10.14341/DM13042
7. Psoma O, Makris M, Tselepis A, Tsimihodimos V. Short-term Glycemic Variability and Its Association With Macrovascular and Microvascular Complications in Patients With Diabetes. J Diabetes Sci Technol. 2024;18(4):956-967. doi: https://doi.org/10.1177/19322968221146808
8. Nusca A, Tuccinardi D, Albano M, et al. Glycemic variability in the development of cardiovascular complications in diabetes. Diabetes Metab Res Rev. 2018;34(8):e3047. doi: https://doi.org/10.1002/dmrr.3047
9. Kovatchev B. Glycemic Variability: Risk Factors, Assessment, and Control. J Diabetes Sci Technol. 2019;13(4):627-635. doi: https://doi.org/10.1177/1932296819826111
10. Mo Y, Lu J, Zhou J. Glycemic variability: Measurement, target, impact on complications of diabetes and does it really matter? J Diabetes Investig. 2024;15(1):5-14. doi: https://doi.org/10.1111/jdi.14112
11. Lazar S, Ionita I, Reurean-Pintilei D, Timar B. How to Measure Glycemic Variability? A Literature Review. Medicina (Kaunas). 2023;60(1):61. doi: https://doi.org/10.3390/medicina60010061
12. Hjort A, Iggman D, Rosqvist F. Glycemic variability assessed using continuous glucose monitoring in individuals without diabetes and associations with cardiometabolic risk markers: A systematic review and meta-analysis. Clin Nutr. 2024;43(4):915-925. doi: https://doi.org/10.1016/j.clnu.2024.02.014
13. Okada K, Hibi K, Gohbara M, et al. Association between blood glucose variability and coronary plaque instability in patients with acute coronary syndromes. Cardiovasc Diabetol. 2015;14:111. doi: https://doi.org/10.1186/s12933-015-0275-3
14. Mita T, Katakami N, Okada Y, et al. Continuous glucose monitoring-derived time in range and CV are associated with altered tissue characteristics of the carotid artery wall in people with type 2 diabetes. Diabetologia. 2023;66(12):2356-2367. doi: https://doi.org/10.1007/s00125-023-06013-3
15. Jadav RK, Yee KC, Turner M, Mortazavi R. Potential Benefits of Continuous Glucose Monitoring for Predicting Vascular Outcomes in Type 2 Diabetes: A Rapid Review of Primary Research. Healthcare (Basel). 2024;12(15):1542. doi: https://doi.org/10.3390/healthcare12151542
16. Lu J, Wang C, Shen Y, et al. Time in Range in Relation to All-Cause and Cardiovascular Mortality in Patients With Type 2 Diabetes: A Prospective Cohort Study. Diabetes Care. 2021;44(2):549-555. doi: https://doi.org/10.2337/dc20-1862
17. Klimontov VV, Saik OV, Korbut AI. Glucose Variability: How Does It Work?. Int J Mol Sci. 2021;22(15):7783. doi: https://doi.org/10.3390/ijms22157783
18. Chehregosha H, Khamseh ME, Malek M, et al. A View Beyond HbA1c: Role of Continuous Glucose Monitoring. Diabetes Ther. 2019;10(3):853-863. doi: https://doi.org/10.1007/s13300-019-0619-1
19. Tokarek J, Budny E, Saar M, et al. Molecular Processes Involved in the Shared Pathways between Cardiovascular Diseases and Diabetes. Biomedicines. 2023;11(10):2611. doi: https://doi.org/10.3390/biomedicines11102611
20. Yin R, Xu Y, Wang X, Yang L, Zhao D. Role of Dipeptidyl Peptidase 4 Inhibitors in Antidiabetic Treatment. Molecules. 2022;27(10):3055. doi: https://doi.org/10.3390/molecules27103055
21. Ussher JR, Greenwell AA, Nguyen MA, Mulvihill EE. Cardiovascular Effects of Incretin-Based Therapies: Integrating Mechanisms With Cardiovascular Outcome Trials. Diabetes. 2022;71(2):173-183. doi: https://doi.org/10.2337/dbi20-0049
22. Andreadi A, Muscoli S, Tajmir R, et al. Recent Pharmacological Options in Type 2 Diabetes and Synergic Mechanism in Cardiovascular Disease. Int J Mol Sci. 2023;24(2):1646. doi: https://doi.org/10.3390/ijms24021646
23. Caturano A, Vetrano E, Galiero R, et al. Advances in the Insulin-Heart Axis: Current Therapies and Future Directions. Int J Mol Sci. 2024;25(18):10173. doi: https://doi.org/10.3390/ijms251810173
24. Rajbhandari J, Fernandez CJ, Agarwal M, Yeap BXY, Pappachan JM. Diabetic heart disease: A clinical update. World J Diabetes. 2021;12(4):383-406. doi: https://doi.org/10.4239/wjd.v12.i4.383
25. Fernandez CJ, Shetty S, Pappachan JM. Diabetic cardiomyopathy: Emerging therapeutic options. World J Diabetes. 2024;15(8):1677-1682. doi: https://doi.org/10.4239/wjd.v15.i8.1677
26. Balogh DB, Wagner LJ, Fekete A. An Overview of the Cardioprotective Effects of Novel Antidiabetic Classes: Focus on Inflammation, Oxidative Stress, and Fibrosis. Int J Mol Sci. 2023;24(9):7789. doi: https://doi.org/10.3390/ijms24097789
27. Dhar A, Venkadakrishnan J, Roy U, et al. A comprehensive review of the novel therapeutic targets for the treatment of diabetic cardiomyopathy. Ther Adv Cardiovasc Dis. 2023;17:17539447231210170. doi: https://doi.org/10.1177/17539447231210170
28. Nikolaidou A, Ventoulis I, Karakoulidis G, et al. Hypoglycemic Drugs in Patients with Diabetes Mellitus and Heart Failure: A Narrative Review. Medicina (Kaunas). 2024;60(6):912. doi: https://doi.org/10.3390/medicina60060912
29. Razavi M, Wei YY, Rao XQ, Zhong JX. DPP-4 inhibitors and GLP-1RAs: cardiovascular safety and benefits. Mil Med Res. 2022;9(1):45. doi: https://doi.org/10.1186/s40779-022-00410-2
30. Ferreira JP, Mehta C, Sharma A, Nissen SE, Rossignol P, Zannad F. Alogliptin after acute coronary syndrome in patients with type 2 diabetes: a renal function stratified analysis of the EXAMINE trial. BMC Med. 2020;18(1):165. doi: https://doi.org/10.1186/s12916-020-01616-8
31. Huang TL, Hsiao FY, Chiang CK, Shen LJ, Huang CF. Risk of cardiovascular events associated with dipeptidyl peptidase-4 inhibitors in patients with diabetes with and without chronic kidney disease: A nationwide cohort study. PLoS One. 2019;14(5):e0215248. doi: https://doi.org/10.1371/journal.pone.0215248
32. Baksh SN, Segal JB, McAdams-DeMarco M, Kalyani RR, Alexander GC, Ehrhardt S. Dipeptidyl peptidase-4 inhibitors and cardiovascular events in patients with type 2 diabetes, without cardiovascular or renal disease. PLoS One. 2020;15(10):e0240141. doi: https://doi.org/10.1371/journal.pone.0240141
33. Lin PJ, Pope E, Zhou FL. Comorbidity Type and Health Care Costs in Type 2 Diabetes: A Retrospective Claims Database Analysis. Diabetes Ther. 2018;9(5):1907-1918. doi: https://doi.org/10.1007/s13300-018-0477-2
34. Wen S, Wang C, Gong M, Zhou L. An overview of energy and metabolic regulation. Sci. China Life Sci. 2018;62:771–790. doi: https://doi.org/10.1007/s11427-018-9371-4
35. Jamison RA, Stark R, Dong J, et al. Hyperglucagonemia precedes a decline in insulin secretion and causes hyperglycemia in chronically glucose-infused rats. Am J Physiol Endocrinol Metab. 2011;301(6):E1174-E1183. doi: https://doi.org/10.1152/ajpendo.00175.2011
36. Mulvihill EE. Dipeptidyl peptidase inhibitor therapy in type 2 diabetes: Control of the incretin axis and regulation of postprandial glucose and lipid metabolism. Peptides. 2018;100:158-164. doi: https://doi.org/10.1016/j.peptides.2017.11.023
37. Ferrannini E. A Journey in Diabetes: From Clinical Physiology to Novel Therapeutics: The 2020 Banting Medal for Scientific Achievement Lecture. Diabetes. 2021;70(2):338-346. doi: https://doi.org/10.2337/dbi20-0028
38. Lv C, Sun Y, Zhang ZY, Aboelela Z, Qiu X, Meng ZX. β-cell dynamics in type 2 diabetes and in dietary and exercise interventions. J Mol Cell Biol. 2022;14(7):mjac046. doi: https://doi.org/10.1093/jmcb/mjac046
39. Chai S, Zhang R, Zhang Y, et al. Effect of dipeptidyl peptidase-4 inhibitors on postprandial glucagon level in patients with type 2 diabetes mellitus: A systemic review and meta-analysis. Front Endocrinol (Lausanne). 2022;13:994944. doi: https://doi.org/10.3389/fendo.2022.994944
40. Hædersdal S, Andersen A, Knop FK, Vilsbøll T. Revisiting the role of glucagon in health, diabetes mellitus and other metabolic diseases. Nat Rev Endocrinol. 2023;19(6):321-335. doi: https://doi.org/10.1038/s41574-023-00817-4
41. Wewer Albrechtsen NJ, Holst JJ, Cherrington AD, et al. 100 years of glucagon and 100 more. Diabetologia. 2023 Aug;66(8):1378-1394. doi: https://doi.org/10.1007/s00125-023-05947-y
42. Monnier L, Colette C, Dunseath GJ, Owens DR. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care. 2007;30(2):263-269. doi: https://doi.org/10.2337/dc06-1612
43. Takebayashi K, Suzuki T, Naruse R, et al. Long-Term Effect of Alogliptin on Glycemic Control in Japanese Patients With Type 2 Diabetes: A 3.5-Year Observational Study. J Clin Med Res. 2017;9(9):802-808. doi: https://doi.org/10.14740/jocmr3118w
44. Su J, Luo Y, Hu S, Tang L, Ouyang S. Advances in Research on Type 2 Diabetes Mellitus Targets and Therapeutic Agents. Int J Mol Sci. 2023;24(17):13381. doi: https://doi.org/10.3390/ijms241713381
45. Huang CJ, Wang WT, Sung SH, et al. Revisiting ‘intensive’ blood glucose control: A causal directed acyclic graph-guided systematic review of randomized controlled trials. Diabetes Obes Metab. 2022;24(12):2341-2352. doi: https://doi.org/10.1111/dom.14819
46. Mohan V, Khunti K, Chan SP, et al. Management of Type 2 Diabetes in Developing Countries: Balancing Optimal Glycaemic Control and Outcomes with Affordability and Accessibility to Treatment. Diabetes Ther. 2020;11(1):15-35. doi: https://doi.org/10.1007/s13300-019-00733-9
47. Shao DW, Zhao LJ, Sun JF. Synthesis and clinical application of representative small-molecule dipeptidyl Peptidase-4 (DPP-4) inhibitors for the treatment of type 2 diabetes mellitus (T2DM). Eur J Med Chem. 2024;272:116464. doi: https://doi.org/10.1016/j.ejmech.2024.116464
48. Lee S, Lee H, Kim Y, Kim E. Effect of DPP-IV Inhibitors on Glycemic Variability in Patients with T2DM: A Systematic Review and Meta-Analysis. Sci Rep. 2019;9(1):13296. doi: https://doi.org/10.1038/s41598-019-49803-9
49. Chai S, Zhang R, Zhang Y, et al. Influence of dipeptidyl peptidase-4 inhibitors on glycemic variability in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne). 2022;13:935039. doi: https://doi.org/10.3389/fendo.2022.935039
50. Yoshikawa F, Uchino H, Nagashima T, et al. Dipeptidyl peptidase-4 inhibitor improves glycemic variability in multiple daily insulin-treated type 2 diabetes: a prospective randomized-controlled trial. Diabetol Int. 2021;13(1):124-131. doi: https://doi.org/10.1007/s13340-021-00513-6
51. Xie Y, Bowe B, Xian H, Loux T, McGill JB, Al-Aly Z. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of major adverse cardiovascular events: emulation of a randomised target trial using electronic health records. Lancet Diabetes Endocrinol. 2023;11(9):644-656. doi: https://doi.org/10.1016/S2213-8587(23)00171-7
52. Koufakis T, Zografou I, Doumas M, Kotsa K. The Current Place of DPP4 Inhibitors in the Evolving Landscape of Type 2 Diabetes Management: Is It Time to Bid Adieu?. Am J Cardiovasc Drugs. 2023;23(6):601-608. doi: https://doi.org/10.1007/s40256-023-00610-8
53. Singh AK, Yadav D, Sharma N, Jin JO. Dipeptidyl Peptidase (DPP)-IV Inhibitors with Antioxidant Potential Isolated from Natural Sources: A Novel Approach for the Management of Diabetes. Pharmaceuticals (Basel). 2021;14(6):586. doi: https://doi.org/10.3390/ph14060586
54. Nauck MA, Quast DR, Wefers J, Pfeiffer AFH. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes Obes Metab. 2021;23 Suppl 3:5-29. doi: https://doi.org/10.1111/dom.14496
55. Chai S, Zhang R, Carr RD, et al. Impact of dipeptidyl peptidase-4 inhibitors on glucose-dependent insulinotropic polypeptide in type 2 diabetes mellitus: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2023;14:1203187. doi: https://doi.org/10.3389/fendo.2023.1203187
56. Nauck MA, Meier JJ. GIP and GLP-1: Stepsiblings Rather Than Monozygotic Twins Within the Incretin Family. Diabetes. 2019;68(5):897-900. doi: https://doi.org/10.2337/dbi19-0005
57. Deacon CF. Metabolism of GIP and the contribution of GIP to the glucose-lowering properties of DPP-4 inhibitors. Peptides. 2020;125:170196. doi: https://doi.org/10.1016/j.peptides.2019.170196
58. Subrahmanyan NA, Koshy RM, Jacob K, Pappachan JM. Efficacy and Cardiovascular Safety of DPP-4 Inhibitors. Curr Drug Saf. 2021;16(2):154-164. doi: https://doi.org/10.2174/1574886315999200819150544
59. Saini K, Sharma S, Khan Y. DPP-4 inhibitors for treating T2DM - hype or hope? an analysis based on the current literature. Front Mol Biosci. 2023;10:1130625. doi: https://doi.org/10.3389/fmolb.2023.1130625
60. Deacon CF. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2020;16(11):642-653. doi: https://doi.org/10.1038/s41574-020-0399-8
61. Love KM, Liu Z. DPP4 Activity, Hyperinsulinemia, and Atherosclerosis. J Clin Endocrinol Metab. 2021;106(6):1553-1565. doi: https://doi.org/10.1210/clinem/dgab078
62. Chen SY, Kong XQ, Zhang KF, Luo S, Wang F, Zhang JJ. DPP4 as a Potential Candidate in Cardiovascular Disease. J Inflamm Res. 2022;15:5457-5469. doi: https://doi.org/10.2147/JIR.S380285
63. Barchetta I, Cimini FA, Dule S, Cavallo MG. Dipeptidyl Peptidase 4 (DPP4) as A Novel Adipokine: Role in Metabolism and Fat Homeostasis. Biomedicines. 2022;10(9):2306. doi: https://doi.org/10.3390/biomedicines10092306
64. Yan Y, Lu H, Zheng Y, Lin S. Association Between Systemic Immune Inflammation Index and Diabetes Mellitus in the NHANES 2003-2018 Population. J Endocr Soc. 2024;8(8):bvae124. doi: https://doi.org/10.1210/jendso/bvae124
65. Tang Y, Feng X, Liu N, et al. Relationship between systemic immune inflammation index and mortality among US adults with different diabetic status: Evidence from NHANES 1999-2018. Exp Gerontol. 2024;185:112350. doi: https://doi.org/10.1016/j.exger.2023.112350
66. Dalle S, Abderrahmani A. Receptors and Signaling Pathways Controlling Beta-Cell Function and Survival as Targets for Anti-Diabetic Therapeutic Strategies. Cells. 2024;13(15):1244. doi: https://doi.org/10.3390/cells13151244
67. Shao S, Xu Q, Yu X, Pan R, Chen Y. Dipeptidyl peptidase 4 inhibitors and their potential immune modulatory functions. Pharmacol Ther. 2020;209:107503. doi: https://doi.org/10.1016/j.pharmthera.2020.107503
68. Huang J, Liu X, Wei Y, et al. Emerging Role of Dipeptidyl Peptidase-4 in Autoimmune Disease. Front Immunol. 2022;13:830863. doi: https://doi.org/10.3389/fimmu.2022.830863
69. Trzaskalski NA, Fadzeyeva E, Mulvihill EE. Dipeptidyl Peptidase-4 at the Interface Between Inflammation and Metabolism. Clin Med Insights Endocrinol Diabetes. 2020;13:1179551420912972. doi: https://doi.org/10.1177/1179551420912972
70. Pechmann LM, Pinheiro FI, Andrade VFC, Moreira CA. The multiple actions of dipeptidyl peptidase 4 (DPP-4) and its pharmacological inhibition on bone metabolism: a review. Diabetol Metab Syndr. 2024;16(1):175. doi: https://doi.org/10.1186/s13098-024-01412-x
71. Rahim K, Shan M, Ul Haq I, et al. Revolutionizing Treatment Strategies for Autoimmune and Inflammatory Disorders: The Impact of Dipeptidyl-Peptidase 4 Inhibitors. J Inflamm Res. 2024;17:1897-1917. doi: https://doi.org/10.2147/JIR.S442106
Об авторах
Л. А. РуяткинаРоссия
Руяткина Людмила Александровна - д.м.н., профессор.
630091, Новосибирск, Красный проспект, д. 52
Конфликт интересов:
Авторы декларируют отсутствие конфликта интересов
Д. С. Руяткин
Россия
Руяткин Дмитрий Сергеевич - к.м.н., доцент.
Новосибирск
Конфликт интересов:
Авторы декларируют отсутствие конфликта интересов
Дополнительные файлы
|
|
1. Рисунок 1. Современные фармакологические варианты лечения сахарного диабета 2 типа и их эффективность в отношении сердечно-сосудистого риска. Адаптировано по Andreadi A. и соавт. (2023) [22]. | |
| Тема | ||
| Тип | Исследовательские инструменты | |
Посмотреть
(544KB)
|
Метаданные ▾ | |
|
|
2. Рисунок 2. Влияние ингибиторов натрий-глюкозного котранспортера 2-го типа, агонистов рецепторов глюкагоноподобного пептида-1 и ингибиторов дипептидилпептидазы-4 на воспаление, окислительный стресс и фиброз. Адаптировано по Balogh D.B. и соавт. (2023) [26]. | |
| Тема | ||
| Тип | Исследовательские инструменты | |
Посмотреть
(449KB)
|
Метаданные ▾ | |
|
|
3. Рисунок 3. Возможные механизмы, лежащие в основе дипептидилпептидазы-4-опосредованного атеросклероза в состоянии инсулинорезистентности. Адаптировано по Love K.M. и Liu Z. (2021) [61]. | |
| Тема | ||
| Тип | Исследовательские инструменты | |
Посмотреть
(709KB)
|
Метаданные ▾ | |
|
|
4. Рисунок 4. Метаболические эффекты и клеточная специфичность дипептидилпептидазы-4 в отношении толерантности к глюкозе и воспаления. Адаптировано по Trzaskalski N.A. и соавт. (2020) [69]. | |
| Тема | ||
| Тип | Исследовательские инструменты | |
Посмотреть
(730KB)
|
Метаданные ▾ | |
Рецензия
Для цитирования:
Руяткина Л.А., Руяткин Д.С. Спектр эффектов ингибиторов дипептидилпептидазы-4: внутри и за пределами гликемического контроля (часть 1). Сахарный диабет. 2025;28(4):404-412. https://doi.org/10.14341/DM13342
For citation:
Ruyatkina L.A., Ruyatkin D.S. Spectrum of effects of dipeptidyl peptidase-4 inhibitors: within and beyond glycemic control (part 1). Diabetes mellitus. 2025;28(4):404-412. (In Russ.) https://doi.org/10.14341/DM13342

Контент доступен под лицензией Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0).















































