Scroll to:
Cardiovascular risk assessment with the American Heart Association calculator in type 2 diabetes patients: A comparison of empagliflozin indications based on American and European guidelines in southeast of Iran in 2023
https://doi.org/10.14341/DM13153
Abstract
BACKGROUND: This study uses the AHA Risk Calculator to initiate empagliflozin in diabetic patients at risk of cardiovascular disease by comparing the threshold to current guidelines.
AIM: The purpose of this study is to use the American Heart Association’s Cardiovascular Risk Calculator to initiate empagliflozin in patients at cardiovascular risk and to determine an appropriate threshold for initiation of this drug compared to current European and American guidelines for the treatment of diabetes mellitus.
MATERIALS AND METHODS: Information was collected on 800 patients with type 2 diabetes mellitus aged 40 to 79 years and aged 40 to 79 years in the city of Kerman. Patients with indications to start empagliflozin were identified in accordance with the latest European and American guidelines. Using the American Heart Association’s 10-year Cardiovascular Disease Risk Calculator, the patients’ scores were calculated.
RESULTS: Of the 800 patients, 435 patients had indications for empagliflozin as recommended. Patients who were recommended to initiate treatment with this medication exhibited a notably elevated likelihood of developing cardiovascular disease. The incidence of risk factors for cardiovascular issues was assessed, with nephropathy, retinopathy, and stroke being the most closely linked to the commencement of empagliflozin as per recommendations in diabetes management guidelines.
CONCLUSION: The American Heart Association calculator can be used to start empagliflozin with a threshold of 6% in women and 6.5% in men.
Keywords
For citations:
Khoshnazar S.M., Shabanzadeh R., Yousefzadeh G. Cardiovascular risk assessment with the American Heart Association calculator in type 2 diabetes patients: A comparison of empagliflozin indications based on American and European guidelines in southeast of Iran in 2023. Diabetes mellitus. 2025;28(2):228-236. https://doi.org/10.14341/DM13153
BACKGROUND
Diabetes is a category of metabolic diseases that is characterized by the occurrence of hyperglycemia in the affected person. The prevalence of this disease in Iran in the studies is about 16 percent, but the prevalence of prediabetes or prediabetes stages is higher than this rate. This disease is divided into two general categories of diabetes type 1 (with higher incidence at an early age and related to impaired insulin production) and type 2 diabetes (with higher prevalence in older age and related to insulin resistance). Over the past two decades, the prevalence of the disease has increased from 30 million in 1985 to 415 million in 2017, and type 2 diabetes has increased due to the increasing prevalence of obesity, decreased physical activity, increasing population age, and industrialization of societies [1].
Diabetes is associated with a number of vascular and non-vascular complications, including infection, skin changes and hearing loss and vascular complications include microvascular (neuropathy, retinopathy and nephropathy) and macrovascular (peripheral vascular disease, stroke and myocardial infarction) [1]. There is a strong linear association between type 2 diabetes and heart failure, which is more closely related to blood sugar levels and insulin resistance [2], so that the risk of heart attack is the same as that of someone who has already had a heart attack [3] and cardiovascular disease is responsible for a substantial majority, specifically 80%, of mortality cases among individuals diagnosed with type 2 diabetes, highlighting the significant impact of this comorbidity on the health outcomes and overall well-being of diabetic patients. They impose a huge amount of money on each country’s health care system every year [4]. Therefore, prevention and early identification and treatment of cardiovascular disease in patients with type 2 diabetes is important, which is not achieved except by proper and timely treatment of type 2 diabetes [5].
Diet, exercise and medication are used to treat diabetes. A suitable diet for diabetes includes all types of micro-brains and the share of each food category such as carbohydrates, proteins and fats with limitation of saturated fats and salts, which should be observed by the patient with direct supervision of nutrition consultant. Moderate intensity exercise of 30 minutes 3 to 4 times per week is recommended. Blood glucose control drugs include insulin and oral medications, which have been found in the trials that have been shown to be two classes of SGLT2 inh among drugs used in diabetes. Metformin and GLP1 RAs are suitable for reducing cardiovascular complications after metformin [6].
SGLT2 inhibitors, such as empagliflozin, dapagliflozin, canagliflozin, and ertugliflozin, have multiple effects. They contribute to weight loss, regulate blood pressure, impact left ventricular filling pressure, and influence albuminuria. However, it’s important to note that these drugs also come with increased risks of urinary tract infections (UTIs), hypotension, diabetic ketoacidosis, acute renal failure, amputation, and fractures [7]. Reduction of cardiovascular complications by this drug class has been evaluated in various clinical trials including EMPA REG, DECLARE TIMI 58 AND CANVAS. In these trials, patients with type 2 diabetes with and without history of cardiovascular complications were evaluated to reduce cardiovascular complications during the use of this drug category, and all three of these trials have suggested the usefulness of these drugs [8]. Most of the effects of these drugs in reducing cardiovascular complications are not related to low blood sugar, but rather the effects of lowering blood pressure and decreasing extravascular volume caused by these drugs [9].
In the field of diabetes treatment, targeted guidelines have been authored that are updated annually. Among these guidelines can be referred to the European and American guidelines in the treatment of diabetes. In the European guideline the start of the pharmaceutical category SGLT2 inhibitors are used in patients with type 2 diabetes. These patients should have pre-existing cardiovascular disease, proteinuria, renal failure, retinopathy, left ventricular hypertrophy, and at least three cardiovascular risk factors (such as obesity, high blood pressure, high blood lipid levels, smoking, and old age). Additionally, if the duration of diabetes is 10 years or more, they should have at least one cardiovascular risk factor for cardiovascular disease. [10]. In the US guidelines, this indication refers to the presence of at least one or more cases of cardiovascular diseases in diabetic patients. These cardiovascular diseases include myocardial infarction, stroke, peripheral vascular disease, angina, heart failure, and renal failure. Specifically, it is defined by either: Proteinuria with albumin excretion greater than 30 mg in 24 hours (normal value below 30), or Reduction of glomerular filtration to below 60 ml per minute, along with high risk factors for heart disease such as retinopathy, left ventricular hypertrophy, obesity, hyperlipidemia, smoking, age, and hypertension [11].
Given the information provided and recognizing the significance of cardiovascular disease as the primary cause of death in diabetic patients, along with the substantial financial burden it places on the country’s health system, it is crucial for physicians and researchers to prioritize addressing this issue. To calculate the risk of cardiovascular disease in diabetic patients more quickly and easily, we can use the American Heart Association’s calculator, which was created by the association in 2013 and edited and rewritten by 2017. And the occurrence of cardiovascular risk is defined as a catheter. The calculator analyzes information related to diabetes history, blood pressure, blood lipids, smoking, age, and sex to estimate the 10-year risk of developing cardiovascular disease [12, 13, 14].
In this project, 800 patients with type 2 diabetes mellitus and the mentioned benefits of SGLT2 inh. 800 patients with T2D will be selected through a questionnaire and through inpatient or clinics in the city. Among these people, a number of those who are based on American and European diabetes guidelines indicate the start of empagliflozin The sensitivity and specificity of the American Heart Association Calculator in determining the appropriate catheter (percentage) for starting the drug empagliflozin compared to the guidelines of the US and Europe is determined so that it can be used as a quantitative alternative to the American and European diabetes guidelines. The individuals diagnosed with type 2 diabetes with the indication of initiation of empagliflozine instead of the guidelines used by the physician and health personnel were used to determine the type 2 diabetes.
RESEARCH AIM
To evaluate the efficacy of the American Heart Association’s Calculator in determining the suitability for initiating empagliflozin therapy in patients with Type 2 Diabetes Mellitus (T2DM), by comparing its sensitivity and specificity against the established American and European diabetes guidelines. This study will involve the selection of 800 patients with T2DM through a comprehensive questionnaire distributed in inpatient settings and clinics within the city. The project seeks to establish whether the Calculator can serve as a reliable quantitative tool, potentially replacing the current guideline-based approach for prescribing empagliflozin, thereby optimizing treatment initiation and improving patient outcomes.
MATERIALS AND METHODS
Our study was conducted as a cross-sectional study in 2013 on patients with type 2 diabetes during April to September 2013. A total of 800 patients with type 2 diabetes (40-79 years old) whose data were collected either in person or absent through a questionnaire designed based on the required data for calculating 10-year cardiovascular risk and the indications of the onset of empagliflozin according to the latest European and American guidelines. Patients were enrolled from Kerman endocrine clinics, hospital archives and hospital endocrine ward. Inclusion criteria were the history of type 2 diabetes, access to blood lipid test and creatinine value, anthropometric data in absentee patients and ages between 40 and 79 years old and exclusion criteria, lack of access to blood lipid information and creatinine value, age outside the age range of 40 to 79 years, and lack of access to anthropometric information in absentee patients. Before entering the study, the conditions were explained to the patients and the patients were informed of confidentiality and non-disclosure of information and not registering their national code and their name and surname.
Place and period of the research
Place of the research. Patients from endocrinology clinics in Kerman city, hospital archive and endocrinology department of the hospital were included in the study.
Period of the research. Our study was conducted as a cross-sectional study in 2013 on patients with type 2 diabetes during April to September 2013.
Populations under study
Population: A total of 800 patients with type 2 diabetes (40-79 years old) whose data were collected either in person or absent through a questionnaire designed based on the required data for calculating 10-year cardiovascular risk and the indications of the onset of empagliflozin according to the latest European and American guidelines.
Inclusion criteria: the history of type 2 diabetes, access to blood lipid test and creatinine value, anthropometric data in absentee patients and ages between 40 and 79 years old.
Exclusion criteria: lack of access to blood lipid information and creatinine value, age outside the age range of 40 to 79 years, and lack of access to anthropometric information in absentee patients. Before entering the study, the conditions were explained to the patients and the patients were informed of confidentiality and non-disclosure of information and not registering their national code and their name and surname.
Method of sampling from the studied population
Using the calculation formula of sample size in cross-sectional studies, considering the accuracy of about 0.05 in the study and prevalence of 23% in previous studies and considering the cross-sectional of this study, the sample size of about 800 people was estimated (p: accuracy in the study, d: prevalence in previous studies and n: Sample size in the study.
n = 1.96² × p(1 – p)∕d²
Study design
The height of the subjects in the study was measured in a standing position without shoes and with a height of 0.1cm with a precision of 0.1 cm. The weight of the patients was measured using a scale with an accuracy of 0.1 kg and with minimal clothing and without shoes. BMI of the subjects in the study was calculated by dividing the weight (kg) by height (m) in two squares and obese subjects were defined as BMI greater than 30. Blood pressure was measured after sitting for at least 10 minutes using standard mercury manometer. If the abnormal blood pressure was measured at the first measurement, the patient’s blood pressure was measured again 30 minutes after the initial measurement. In absent patients (hospital archives), this information was extracted based on the data recorded in the hospitalization records.
The criteria for smoking was autobiographical from current smoking by the patient. The age of the patient was considered as intervals (years) at the time of inclusion. The duration of diabetes was considered as the interval from laboratory diagnosis of diabetes to the time of inclusion in the study.
Blood lipid profile and creatinine were considered based on the closest periodic checkup tests after 12 hours of fasting or data recorded in the patients’ clinical records. GFR was calculated using cockroft-gault formula with age (years), weight (kgr), sex and creatinine (mgr/dl) and less than 60 ml/min was considered as diabetic nephropathy. In patients with albumin excretion test in urine, excretion of albumin greater than 30 mgr/dl was considered as diabetic nephropathy.
Diabetic retinopathy was defined by questioning the patients about periodic ophthalmology visits and the presentation of retinal and vascular involvement in diabetes by ophthalmologist with or without interventional ophthalmology.
History of taking lipids or blood pressure pills were collected from the patients’ medical history. The history of limb vascular failure was based on the occurrence of limp and pain during walking or activity and previous history of myocardial infarction or preceding in the patient or paraclinical evidence of ischemia and vascular failure due to diabetes.
According to the European guidelines the start of the drug category SGLT2 inh. In type 2 diabetes patients, pre-existing cardiovascular disease (cerebrovascular failure), protein uria (albuminuria more than 30 mgr/dl), Kidney dysfunction (indicated by a glomerular filtration rate (GFR) less than 60 ml/min), retinal complications (detected during ophthalmology examinations due to diabetes), enlargement of the left ventricle of the heart (observed via echocardiography), and the presence of at least three cardiovascular risk factors (obesity (BMI more than 30 kgr/m²), high blood pressure (more than 130/90 mmhg) High blood lipids (blood lipid values based on the he existence or absence of risk factors for cardiovascular disease), current smoking and older age (over 50 years) or duration of diabetes 10 years and more, along with at least one cardiovascular risk factor for heart failure disease.
According to US guidelines, the presence of one or more cases of cardiovascular diseases in diabetic patients includes conditions such as: myocardial infarction, stroke, peripheral vascular disease, angina, heart and renal failure (indicated by proteinuria with albumin excretion greater than 30 mg in 24 hours or a reduction in glomerular filtration rate below 60 ml per minute) Additionally, individuals at high risk for cardiovascular diseases may exhibit: retinopathy (detected during ophthalmic examinations due to diabetes), left ventricular hypertrophy (observed via echocardiography), obesity (BMI greater than 30 kg/m²), hyperlipidemia (based on blood lipid values and the presence or absence of cardiovascular disease risk), current smoking, age (more than 50 years), hypertension (blood pressure exceeding 130/90 mmHg).
According to the American Heart Association, calculating the 10-year risk of cardiovascular disease involves considering data such as age (in years), sex, systolic blood pressure (mmHg), and diastolic blood pressure (mmHg), total blood cholesterol (mgr/dl), HDL (mgr/dl), LDL (mgr/dl), history of diabetes, current smoking, treatment of hypertension and treatment of blood lipids were entered into this calculation. Finally, the response was determined as a percentage of risk (low risk (under 5%), boundary risk (between 5% and 7.4%), medium risk (between 7.5% and 19.9%) and high risk (more than 20%).
Methods
Specify the definition of including and excluding criteria of observations. For example, if you excluded patients with liver pathology, specify which documents or examinations you used to do this.
List all the clinical, laboratory, instrumental, and other indicants under study which results are provided in the article. Specify or describe the evaluation methods for each of them. For clinical diagnoses, their forms, stages, complications, relapses, remissions, and other clinical events provide the criteria for determining them (or links to such criteria). For laboratory and instrumental indicators, specify the names of the methods and the equipment used.
When you describe experimental research, specify the main indicant (outcome) by which the effect of the intervention is evaluated and describe its determinative criteria.
Statistical analysis
Data were analyzed using SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA). Quantitative variables were reported as standard deviation ± mean and qualitative data as number and percentage. The Chi-square test was employed to compare qualitative variables among multiple groups, while the t-independent test was utilized to compare quantitative variables between the two groups. Univariate logistic regression was employed to determine the factors influencing the outcome. The role of significant P-value variables in the regression model in predicting unhealthy metabolic status through receiver operating characteristic (ROC) curve was investigated using MedCalc Statistical Software version 20.013 (MedCalc® Software Ltd, Ostend, Belgium). Optimal cut-off was determined using the Youden index. P<0.05 was considered significant.
Ethics review
The study protocol was fully confirmed by the ethical committee (IR.KMU.AH.REC.1401.179) and 401000321 code. The study design is cross-sectional and doesn’t need to registration number.
RESULTS
In a cross-sectional and retrospective study carried out between April and September 2002, researchers collected data from 800 patients with type 2 diabetes, aged 40-79 years. The data were obtained through a questionnaire administered at Kerman endocrine clinics, as well as from hospital archives and the endocrinology ward. Frequency of obesity in patients with type 2 diabetes, nephropathy, retinopathy, hyperlipidemia, current smoking use, cardiovascular disease, stroke, peripheral arterial disease and hypertension are shown in (Table 1).
Table 1. Frequency of cardiovascular risk factors among individuals with type 2 diabetes
|
Percent |
Frequency |
||
|
14.6 |
117 |
Yes |
Obesity |
|
85.4 |
683 |
No |
|
|
27 |
216 |
Yes |
Nephropathy |
|
73 |
584 |
No |
|
|
12.9 |
103 |
Yes |
Retinopathy |
|
87.1 |
697 |
No |
|
|
29.4 |
235 |
Yes |
Hyperlipidemia |
|
70.6 |
565 |
No |
|
|
13.9 |
111 |
Yes |
Current smoking |
|
86.1 |
689 |
No |
|
|
11.5 |
92 |
Yes |
Cardiovascular disease |
|
88.5 |
708 |
No |
|
|
6 |
48 |
Yes |
Stroke |
|
94 |
752 |
No |
|
|
9.9 |
79 |
Yes |
Peripheral arterial disease |
|
90.1 |
721 |
No |
|
|
31 |
248 |
Yes |
High blood pressure |
|
69 |
552 |
No |
|
The average LDL, HDL, cholesterol, and Systolic and diastolic blood pressure is shown in (Table 2).
Table 2. Determination of mean lipid profile and mean systolic and diastolic blood pressure in patients with type 2 diabetes
|
Standard deviation |
Mean |
|
|
4.23 |
39.110 |
LDL |
|
11.87 |
38.66 |
HDL |
|
55.07 |
179.91 |
Total cholesterol |
|
14.87 |
128.25 |
Systolic blood pressure |
|
11.27 |
80.69 |
Diastolic blood pressure |
According to the latest American and European diabetes guidelines, 435 out of 800 patients had indication to start empagliflozin so the prevalence of patients with empagliflozin was 54.4%.
Data from patients entered the American Heart Association’s 10-year cardiovascular risk calculator in patients who were indicated to start empagliflozin, the majority (49.9%) had a high risk of cardiovascular disease, while in those without an indication for empagliflozin, the majority (46.8%) had a low risk. The difference in cardiovascular disease risk frequency was significant between the two groups (p=0.001). Patients with an indication for empagliflozin had a significantly higher mean 10-year risk of cardiovascular disease (22.17%) compared to those without an indication (10.67%), and this difference was statistically significant (p-value=0.001) (Table 3).
Table 3. Frequency and average of 10-year risk of cardiovascular disease in patients with type 2 diabetes with and without indication of starting empagliflozin
|
p-value |
No indication for empagliflozin |
Indication for empagliflozin |
||
|
0.001 |
171 (46.8%) |
34 (7.8%) |
Low |
Frequency 10-year risk of cardiovascular disease |
|
36 (9.9%) |
27 (6.2%) |
Border |
||
|
95 (26%) |
157 (36.1%) |
Medium |
||
|
63 (17.3%) |
217 (49.9%) |
Much |
||
|
0.001 |
10.67±0.6 |
22.17±0.67 |
Average 10-year risk of cardiovascular disease |
|
The risk was 6% according to the American Heart Association’s 10-year cardiovascular risk calculator with sensitivity of 97.8% and specificity of 98.5% in accordance with Figure 1 (the area under the curve is 0.77 and was significant (p>0.05) and was determined as the catheter of the onset of amglyflozin in patients with type 2 diabetes.
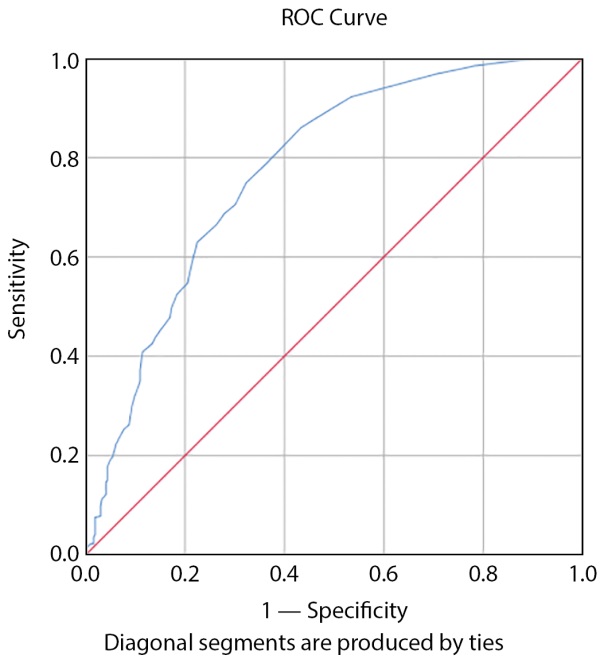
Figure 1. Cutoff Beginning Empagliflozine Based on 10-Year Risk of Cardiovascular Disease According to the American Heart Association Calculation.
The risk was 6% according to the American Heart Association’s 10-year cardiovascular risk calculation with sensitivity of 97.3% and specificity of 98.3% in women according to Figure 2 (the area under the curve is 0.77 and was significant (p>0.05), and the risk was 6.5% with sensitivity of 97.3% and specificity of 98.3% in men according to Figure 3 (the area under the curve was 0.87 and significant (p>0.05) and (Table 4) as the starting catheter Amogliflozine was determined based on gender in patients with type 2 diabetes.
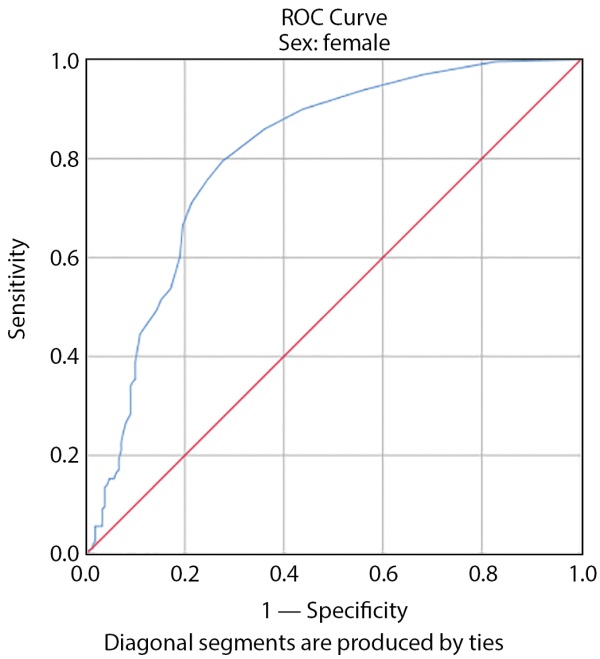
Figure 2. Cutoff Beginning of Empagliflozine Based on 10-Year Risk of Cardiovascular Disease According to the American Heart Association Calculation in Women.
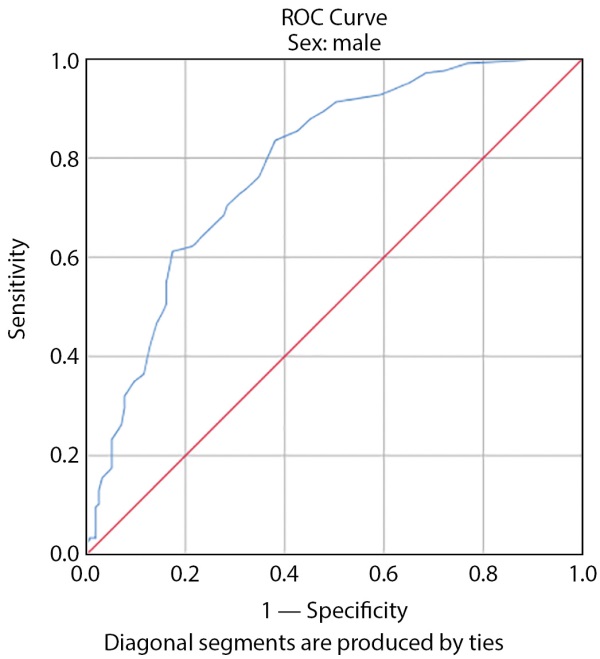
Figure 3. Cutoff Beginning Empagliflozine Based on 10-Year Risk of Cardiovascular Disease According to American Heart Association Calculation in Men.
Table 4. Determination of the Cataf Indication of Starting Empagliflozine Based on 10-Year Risk Percentage of Cardiovascular Disease by Gender in individuals with Type 2 Diabetes
|
confidence interval 95% |
p-value |
standard error |
Area under the curve |
Gender |
|
|
Upper limit |
Lower limit |
||||
|
0.9 |
0.67 |
0.02 |
0.05 |
0.78 |
Man |
|
0.82 |
0.71 |
<0.001 |
0.02 |
0.77 |
Woman |
According to univariate analysis of cardiovascular risk factors studied, age, duration of diabetes, nephropathy, retinopathy, hyperlipidemia, current smoking, cardiovascular disease, stroke, peripheral arterial disease and hypertension were significantly correlated with the indication of empagliflozin initiation and the highest effects were nephropathy (85.79%), retinopathy (36.02%) and stroke (10.15%), respectively (Table 5).
Table 5. Determination of Regression Regression Indication of the Onset of Empagliflozine Based on Cardiovascular Factor Risk in individuals with Type 2 Diabetes
|
OR |
p-value |
|
|
1.21 |
0.16 |
Gender |
|
0.86 |
<0.001 |
Age |
|
1.15 |
0.4 |
Obesity |
|
1 |
96/0 |
LDL |
|
1.01 |
0.09 |
HDL |
|
1 |
0.064 |
Cholesterol |
|
0.77 |
<0.001 |
Duration of diabetes |
|
85.79 |
<0.001 |
Nephropathy |
|
36.02 |
<0.001 |
Retinopathy |
|
2.87 |
<0.001 |
Hyperlipidemia |
|
1.90 |
0.003 |
Smoking |
|
12.42 |
<0.001 |
Cardiovascular disease |
|
10.12 |
<0.001 |
Stroke |
|
18.8 |
<0.001 |
Peripheral arterial disease |
|
4.49 |
<0.001 |
High blood pressure |
DISCUSSION
In our study, 800 patients with type 2 diabetes whose mean age was 9.97±58.47 years with the age range (88-40 years) were studied. Of these, 361 (45.1%) were male and 439 (54.9%) were female. The mean duration of type 2 diabetes was 11.5±7.48 years.
In the EMPRISE study, 18,800 people took empagliflozin and 201839 took sitagliptin. Compared to the sitagliptin-treated group, patients receiving empagliflozin treatment had fewer underlying diseases, were younger, and had a higher proportion of males. The average age of the patients was 59 years, and 54% of them were male. Additionally, 25% of the patients had a history of cardiovascular disease. The prevalence of diabetic nephropathy was 7.9%, diabetic retinopathy was 5.9% and peripheral vascular disease was 4%. 76.4% had hypertension and 6.7% had chronic kidney disease, mean total serum cholesterol was 17669, the average HDL was 44.07 and the average LDL was 84.41 [15].
In the EMPA REG study, 7042 participants, the mean age of patients was 63 years.
72% of the participants were male. More than 50% of patients had a diabetes duration of more than 10 years. 47% had a history of myocardial infarction. 23% had a history of stroke. 52% of patients had GFR between 60 and 90. 77% were treated with hyperlipidemia and 94% were treated with hypertension. 20% of patients were obese, mean blood pressure was 135.77, serum total cholesterol was 162.4, LDL 85 and HDL was 46.4 [16][17].
The CANVAS study involved 10,142 patients who had type 2 diabetes mellitus and were over 50 years of age. These patients had at least 2 cardiovascular risk factors and were enrolled in a randomized, double-blind clinical trial. One group was treated with placebo and the other was treated with canagliflozin at a dose of 100 mgr or 300 mgr and followed for 2.4 years. In these patients, the average GFR was 76.5±20.5 ml/min/1.73 m², and the duration of diabetes was 13.5 years on average. Cases of cardiovascular death, non-fatal myocardial infarction and non-fatal stroke during this period were reported 26.9 cases per 1,000 patients per year in the canagliflozin-treated group and 31.5 cases per 1,000 patients per year in the placebo-treated group. Hospitalizations for heart failure were reported to be 5.5 cases per 1,000 patients per year in the canagliflozin treated group and 8.7 cases per 1,000 patients per year in the placebo-treated group [18].
The DECLARE-TIMI 58 study included 7,160 patients with type 2 diabetes. These patients were men over 55 years and women over 60 years old, all of whom had at least one cardiovascular risk factor. They were randomly enrolled in a double-blind clinical trial. One group was treated with placebo and the other group received 10 mgr dapagliflozin and followed up for 4 years. The average GFR was 85.25 ml/min/1.73 m² in these patients, the average duration of diabetes was 11 years. Cardiovascular mortality, non-fatal myocardial infarction and non-fatal stroke were 8.8% in the dapagliflozin treated group and 9.4% in placebo-treated group. Hospitalizations due to heart failure were 2.5% in the group treated with dapagliflozin and 3.3% in placeo-treated group [19].
The Salsali et al. study was conducted as a meta-analysis of data from 8 clinical trials of 11,292 patients with type 2 diabetes. The patients were divided into two groups treated with empagliflozin (10 mgr and 25 mgr) and placebo therapy. In the group treated with empagliflozin, there was a 4-point MACE (Major Adverse Cardiovascular Events) which included cardiovascular mortality, non-fatal myocardial infarction, non-fatal stroke, and unstable angina with hospitalization. This occurred in 9.5% of the patients. In comparison, the placebo-treated group had an 8% occurrence of the same events. Additionally, the empagliflozin-treated group experienced a 3-point MACE, which included cardiovascular mortality, non-fatal myocardial infarction, and non-fatal stroke. This was in contrast to the placebo-treated group. [20].
In our study, in accordance with the latest American and European diabetes guidelines, 435 (54.4%) of 800 people indicated the onset of empagliflozin. Among patients recommended to begin empagliflozin treatment, the majority at high cardiovascular risk numbered 217 (49.9%). Conversely, in the group not advised to start empagliflozin, most individuals were at low cardiovascular risk. The average 10-year cardiovascular risk was 22.17% for those suggested to start empagliflozin, compared to 10.67% for those without such a recommendation. These findings were statistically significant.
Based on prior research and the advantages of empagliflozin in lessening cardiovascular issues among type 2 diabetes patients, current protocols have pinpointed the criteria for commencing this medication in those with a significant cardiovascular threat. This disparity is attributed to the cardiovascular peril of patients outlined in these protocols as a criterion for initiating treatment (references 10 and 11). Earlier studies have not assessed the decade-long cardiovascular risk for patients with and without the recommendation to initiate empagliflozin therapy.
In our study, age, duration of diabetes, nephropathy, retinopathy, hyperlipidemia, current smoking, cardiovascular disease, stroke, peripheral arterial disease and hypertension were significantly correlated with the indication of starting empagliflozin and the highest effect was nephropathy (85.79%), retinopathy (36.02%) and stroke (10.15%).
The risk was 6% according to the American Heart Association’s 10-year cardiovascular risk calculation with sensitivity of 97.8% and specificity of 98.5% as the catheter of amoglyflozin in Individuals diagnosed with type 2 diabetes. The risk was 6% according to the American Heart Association’s 10-year cardiovascular risk calculation with sensitivity of 97.3%, specificity of 98.3% in women and 6.5% with sensitivity of 97.3% and specificity of 98.3% in men as the catheter of starting amoglyflozin in individuals diagnosed with type 2 diabetes was determined based on gender. Among the limitations of our study was the lack of access to all patient data, such as information about blood lipid tests, creatinine number and albumin excretion in urine. To solve this problem, studies by performing the required experiments simultaneously with obtaining data for better access to these experiments are recommended. One of the strengths of our study is the importance of calculating 10-year cardiovascular risk as an alternative to starting empagliflozin in patients with type 2 diabetes and determining the appropriate percentage for starting this drug due to the benefits of this drug in reducing future cardiovascular events.
CONCLUSION
According to our study, patients with the indication of starting empagliflozin according to European and American diabetes guidelines have a higher risk of cardiovascular disease based on the American Heart Association’s 10-year cardiovascular risk calculator and this is statistically significant. Considering this issue in patients with type 2 diabetes, this calculator can be used as a suitable alternative to these guidelines for starting empagliflozin. According to our study, a 6% cataf with high sensitivity and specificity indicates the onset of empagliflozin.
OTHER INFORMATION
The source of financing. No funding was received by the researchers for this research.
Conflicts of interests. The researchers declare that no conflicts of interest exist for this research work.
Participation of authors. Gholamreza Yousefzadeh: Conceptualization, methodology, Investigation, Project administration. Reza Shabanzadeh: formal analysis. Seyedeh Mahdieh Khoshnazar: Writing – original draft and Writing – review & editing. All authors have read and agreed to the published version of the manuscript. “All the authors approved the final version of the article before the publication and expressed their consent to be responsible for all aspects of the work, which implies proper investigation and resolving of issues related to the accuracy or integrity of any part of the work.”
Acknowledgments. The authors would like to thank Kerman University of Medical Sciences for concerning this manuscript.
References
1. Kasper D, Fauci A, Hauser S, et al. Harrison’s Principles of Intrenal Medicine: Diabetes Mellitus. New York: Mcgraw-Hill; 2015.
2. Paneni F, Lüscher TF. Cardiovascular Protection in the Treatment of Type 2 Diabetes: A Review of Clinical Trial Results Across Drug Classes. Am J Med. 2017;130(6S):S18-S29. doi: https://doi.org/10.1016/j.amjmed.2017.04.008
3. Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108(12):1527-32. doi: https://doi.org/10.1161/01.CIR.0000091257.27563.32
4. Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, Piegas L, Calvin J, Keltai M, Budaj A. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation. 2000;102(9):1014-9. doi: https://doi.org/10.1161/01.cir.102.9.1014
5. Mellbin LG, Anselmino M, Rydén L. Diabetes, prediabetes and cardiovascular risk. Eur J Cardiovasc Prev Rehabil. 2010;17 Suppl 1:S9-14. doi: https://doi.org/10.1097/01.hjr.0000368192.24732.2f
6. American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2020 [published correction appears in Diabetes Care. 2020 Aug;43(8):1979. doi: https://doi.org/10.2337/dc20-ad08a.]. Diabetes Care. 2020;43(Suppl 1):S98-S110. doi: https://doi.org/10.2337/dc20-S009
7. Lu H, Meyer P, Hullin R. Use of SGLT2 inhibitors in cardiovascular diseases: why, when and how?. Swiss Med Wkly. 2020;150:w20341. doi: https://doi.org/10.4414/smw.2020.20341
8. Canivell S, Mata-Cases M, Vlacho B, et al. How Many Patients with Type 2 Diabetes Meet the Inclusion Criteria of the Cardiovascular Outcome Trials with SGLT2 Inhibitors? Estimations from a Population Database in a Mediterranean Area. J Diabetes Res. 2019;2019:2018374. doi: https://doi.org/10.1155/2019/2018374
9. Abdul-Ghani M, Del Prato S, Chilton R, DeFronzo RA. SGLT2 Inhibitors and Cardiovascular Risk: Lessons Learned From the EMPA-REG OUTCOME Study. Diabetes Care. 2016;39(5):717-725. doi: https://doi.org/10.2337/dc16-0041
10. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD [published correction appears in Eur Heart J. 2020 Dec 1;41(45):4317. doi: https://doi.org/10.1093/eurheartj/ehz828.]. Eur Heart J. 2020;41(2):255-323. doi: https://doi.org/10.1093/eurheartj/ehz486
11. American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2022 [published correction appears in Diabetes Care. 2022 May 1;45(5):1296. doi: https://doi.org/10.2337/dc22-er05.] [published correction appears in Diabetes Care. 2022 Sep 01;45(9):2178-2181. doi: https://doi.org/10.2337/dc22-ad08.]. Diabetes Care. 2022;45(Suppl 1):S144-S174. doi: https://doi.org/10.2337/dc22-S010
12. Bertoluci MC, Rocha VZ. Cardiovascular risk assessment in patients with diabetes [published correction appears in Diabetol Metab Syndr. 2017 Sep 19;9:70. doi: https://doi.org/10.1186/s13098-017-0270-9.]. Diabetol Metab Syndr. 2017;9:25. doi: https://doi.org/10.1186/s13098-017-0225-1
13. Das SR, Everett BM, Birtcher KK, et al. 2020 Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients With Type 2 Diabetes: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117-1145. doi: https://doi.org/10.1016/j.jacc.2020.05.037
14. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in Circulation. 2019;140(11):e649-e650. doi: https://doi.org/10.1161/CIR.0000000000000725.] [published correction appears in Circulation. 2020 Jan 28;141(4):e60. doi: https://doi.org/10.1161/CIR.0000000000000755.] [published correction appears in Circulation. 2020 Apr 21;141(16):e774. doi: https://doi.org/10.1161/CIR.0000000000000771.]. Circulation. 2019;140(11):e596-e646. doi: https://doi.org/10.1161/CIR.0000000000000678
15. Patorno E, Pawar A, Franklin JM, et al. Empagliflozin and the Risk of Heart Failure Hospitalization in Routine Clinical Care. Circulation. 2019;139(25):2822-2830. doi: https://doi.org/10.1161/CIRCULATIONAHA.118.039177
16. Kluger AY, Tecson KM, Barbin CM, et al. Cardiorenal Outcomes in the CANVAS, DECLARE-TIMI 58, and EMPA-REG OUTCOME Trials: A Systematic Review. Rev Cardiovasc Med. 2018;19(2):41-49. doi: https://doi.org/10.31083/j.rcm.2018.02.907
17. Fitchett D, Butler J, van de Borne P, et al. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME® trial. Eur Heart J. 2018;39(5):363-370. doi: https://doi.org/10.1093/eurheartj/ehx511
18. Mahaffey KW, Neal B, Perkovic V, et al. Canagliflozin for Primary and Secondary Prevention of Cardiovascular Events: Results From the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation. 2018;137(4):323-334. doi: https://doi.org/10.1161/CIRCULATIONAHA.117.032038
19. Wiviott SD, Raz I, Bonaca MP, et al. The design and rationale for the Dapagliflozin Effect on Cardiovascular Events (DECLARE)-TIMI 58 Trial. Am Heart J. 2018;200:83-89. doi: https://doi.org/10.1016/j.ahj.2018.01.012
20. Salsali A, Kim G, Woerle HJ, Broedl UC, Hantel S. Cardiovascular safety of empagliflozin in patients with type 2 diabetes: a meta-analysis of data from randomized placebo-controlled trials. Diabetes Obes Metab. 2016;18(10):1034-1040. doi: https://doi.org/10.1111/dom.12734
21. Zinman B, Inzucchi SE, Lachin JM, et al. Rationale, design, and baseline characteristics of a randomized, placebocontrolled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOME™). Cardiovasc Diabetol. 2014;13:102. doi: https://doi.org/10.1186/1475-2840-13-102
About the Authors
Seyedeh Mahdieh KhoshnazarIslamic Republic of Iran
Seyedeh Mahdieh Khoshnazar, PhD, Associated Professor
Haft Bagh Alavi Street
Reza Shabanzadeh
Islamic Republic of Iran
Reza Shabanzadeh, MD
Haft Bagh Alavi Street
Gholamreza Yousefzadeh
Islamic Republic of Iran
Gholamreza Yousefzadeh, MD
Haft Bagh Alavi Street
Supplementary files
|
|
1. Figure 1. Cutoff Beginning Empagliflozine Based on 10-Year Risk of Cardiovascular Disease According to the American Heart Association Calculation. | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(204KB)
|
Indexing metadata ▾ | |
|
|
2. Figure 2. Cutoff Beginning of Empagliflozine Based on 10-Year Risk of Cardiovascular Disease According to the American Heart Association Calculation in Women. | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(211KB)
|
Indexing metadata ▾ | |
|
|
3. Figure 3. Cutoff Beginning Empagliflozine Based on 10-Year Risk of Cardiovascular Disease According to American Heart Association Calculation in Men. | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(118KB)
|
Indexing metadata ▾ | |
Review
For citations:
Khoshnazar S.M., Shabanzadeh R., Yousefzadeh G. Cardiovascular risk assessment with the American Heart Association calculator in type 2 diabetes patients: A comparison of empagliflozin indications based on American and European guidelines in southeast of Iran in 2023. Diabetes mellitus. 2025;28(2):228-236. https://doi.org/10.14341/DM13153

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0).















































