Scroll to:
Diabetes Risk Screening Tools for Prediabetes: A Comprehensive Scoping Review of Evidence and Implementation
https://doi.org/10.14341/DM13324
Abstract
BACKGROUND/OBJECTIVES: Diabetes risk screening tools are essential for identifying individuals with prediabetes and preventing the progression to diabetes. However, systematic reviews focusing on such tools, particularly for prediabetes screening, are scarce. This scoping review examines the characteristics, development methods, and effectiveness of diabetes risk assessment tools in identifying prediabetes and predicting its progression to diabetes.
MATERIALS AND METHODS: A scoping review was conducted following the Joanna Briggs Institute methodology. Searches were performed in PubMed, ScienceDirect, and Google Scholar, complemented by citation tracking. Eligible studies included asymptomatic adults with prediabetes. Studies were excluded if they lacked relevant data, were not in English, or had no validation measures. Data were extracted independently by two reviewers and synthesized narratively, focusing on study design, risk model features, performance statistics, and quality assessments.
RESULTS: Fourteen studies met the inclusion criteria, covering 26 risk models. Sensitivity and specificity were used in 9 risk screening tools, with Hazard Ratios and C-Statistics assessing diabetes progression in six. Common risk factors included age, BMI, family history of diabetes, and hypertension. Non-invasive tools and predictive models showed promise, with most studies assessed as having a low risk of bias using QUADAS-2. High-sensitivity tools utilizing FBG, HbA1c, and OGTT cutoffs demonstrated effectiveness but require balancing cost and feasibility for broader implementation.
CONCLUSION: A range of different screening tools has been tested that could identify people with prediabetes or a high risk of developing type 2 diabetes. However, where sufficient evidence was available to compare tools across studies the performance of these tools was inconsistent. Several tools have only been investigated in single studies, with uncertainty around their wider generalisability. Clinicians or researchers wishing to screen people for prediabetes or a high risk of developing type 2 diabetes using any of these tools should be aware of their potential limitations.
The full text of the article is available in the electronic version of the journal on the website www.dia-endojournals.ru
For citations:
Wongrith P., Dangkrajang S., Nam T. Diabetes Risk Screening Tools for Prediabetes: A Comprehensive Scoping Review of Evidence and Implementation. Diabetes mellitus. 2025;28(4):348-358. https://doi.org/10.14341/DM13324
INTRODUCTION
The global rise in type 2 diabetes (T2DM) has profoundly impacted healthcare systems, especially in low- and middle-income countries where over 75% of diabetes cases occur [1].
Prediabetes — a condition marked by blood sugar levels higher than normal but not yet at diabetic levels — is a critical precursor to T2DM, often progressing to full-blown diabetes if left unaddressed [2][3]. Individuals with prediabetes face heightened risks for complications like nephropathy, neuropathy, and macrovascular diseases [3–5]. Studies show that up to 70% of those with prediabetes eventually develop T2DM, sometimes within five years [2][6]. The American Diabetes Association (ADA) defines prediabetes using HbA1c levels between 5.7% and 6.4% or fasting plasma glucose levels between 100 and 125 mg/dL [7–11]. However, there is no universal consensus on the HbA1c range that best identifies high-risk individuals, as different recommendations vary across organizations [12][13]. In the Asia-Pacific region, guidelines advocate for screening and intervention for those aged 35 and older or individuals at high risk, using laboratory tests such as FPG, HbA1c, and the 75-gram OGTT to support early detection and potential prevention of T2DM [14]. Despite these screening protocols, diabetes risk assessment tools differ significantly in sensitivity, specificity, and suitability across diverse populations [9][15–18].Previous research identifies several key risk factors for prediabetes including sex (female) [19–22], age (45 years or older) [19][21][23][24], Body Mass Index (overweight or obese) [21][25], waist circumference and family history of diabetes [26], high blood pressure [22], polycystic ovary syndrome in women [27][28], dyslipidemia [29], psychological factors such as stress or depression [6], lifestyle behaviors like smoking or tobacco use and alcohol consumption [22][30], poor dietary habits [28][31–33], and physical inactivity [34]. This scoping review aims to summarise existing research on diabetes risk screening tools systematically, identifying knowledge gaps to support potential shifts toward population-wide screening within community-based programs. Specifically, this review examined the characteristics, development methods, and effectiveness of diabetes risk assessment tools for identifying prediabetes and monitoring progression to diabetes in at-risk individuals.
MATERIALS AND METHODS
Literature Review Strategy
This review systematically identified and synthesized studies on diabetes risk assessment tools (Fig. 1). The methodology followed PRISMA-ScR guidelines, accessible at http://www.prisma-statement.org/ and https://www.prisma-statement.org/scoping [35] (see PRISMA checklist in the supplementary file). The protocol was reviewed and revised with input from the advisory board of the Community Medicine Division at Thammasat University; a Principal Research fellow from Southampton Health Technology Assessments Centre (SHTAC), School of Healthcare Enterprise and Innovation, University of Southampton, UK; and members of the research team.
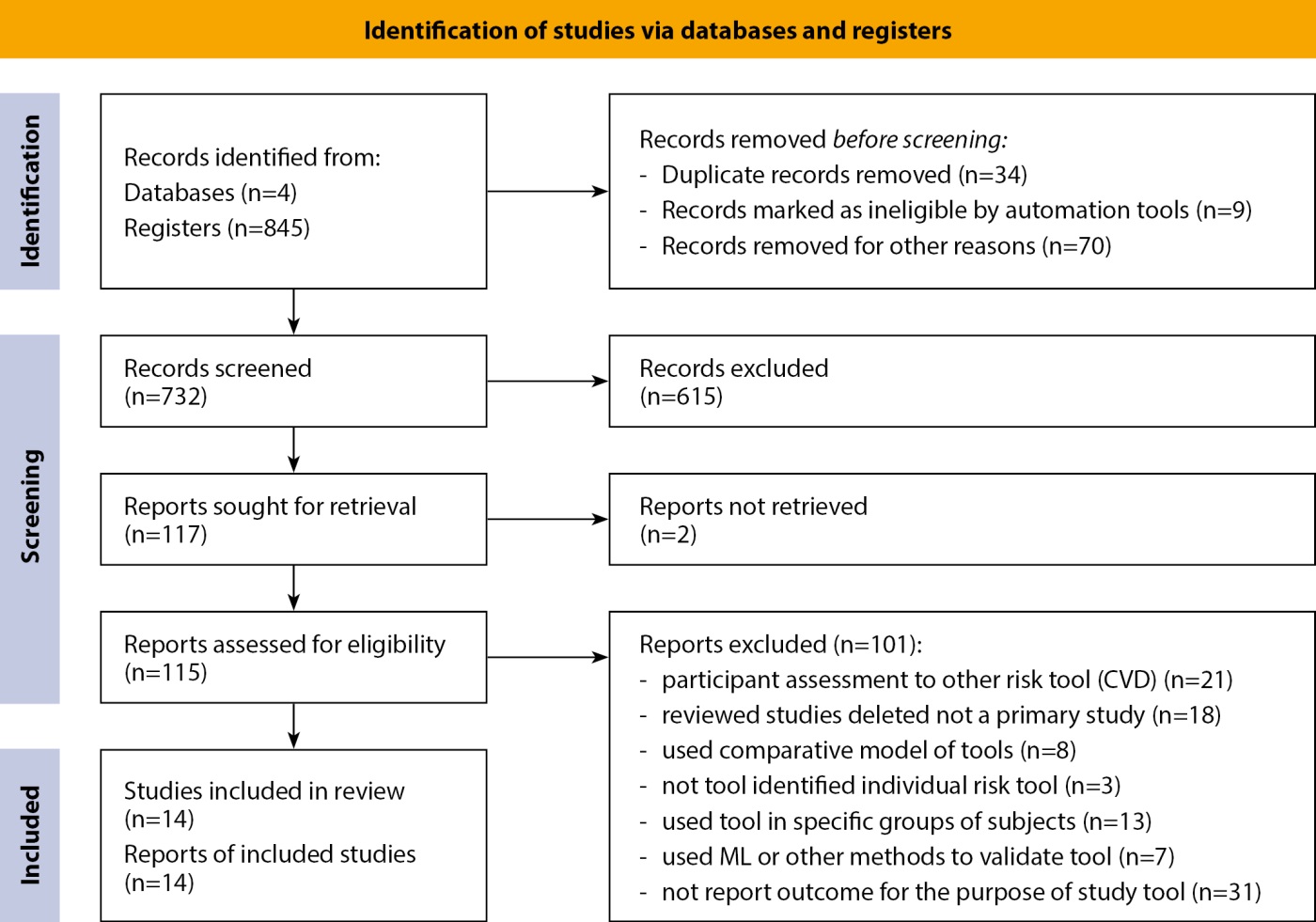
Figure 1. PRISMA 2020 flow diagram.
Eligibility Criteria:
1) Inclusion: Studies on diabetes risk tools for adults with prediabetes or T2DM risks (18+), examining factors affecting tool adoption, implementation, or validation.
2) Exclusion: Studies lacking relevant data, focusing on genetic factors, or not classified as original research. Also excluded were studies focusing on children, pregnant individuals, or unrelated conditions.
Search Strategy and Study Selection
A systematic search across PubMed, ScienceDirect, and Google Scholar used Boolean operators, MeSH terms, and synonyms (e.g., "diabetes risk assessment", "prediabetes screening", "adults", "non-invasive tools") (Appendix A, B). The timeframe covered October 2012 to September 30, 2022, and October 1, 2022 to September 2024.
Studies were selected through:
Title & Abstract Screening: Based on predefined criteria.
Full-Text Review: To confirm eligibility.
Data Extraction & Synthesis
Two reviewers independently extracted data using a standardized QUADAS-2 form. Discrepancies were resolved by a third reviewer. Of 843 identified studies, 14 met inclusion criteria (see Figure 1 for the PRISMA flow chart summarising the selection process). These studies included 26 risk models (e.g., THIARISK, CANRISK, FINDRISC, ADA-Risk). Data on AU-ROC, sensitivity, specificity, and validation efforts were summarized in tabular format for comparison.
Risk of Bias Assessment
As this is a scoping review, for which risk of bias assessment is regarded by JBI as optional rather than mandatory [36], we used a pragmatic approach to the risk of bias assessment. That is, we selected a subset of what we considered to be the most important risk of bias questions from a relevant risk of bias tool for screening studies. The QUADAS-2 tool [37][38], was used to assess bias across four domains: Patient Selection, Index Test, Reference Standard, and Flow and Timing. For comparative accuracy, QUADAS-C [39], was applied where relevant. Additional quality considerations included sampling and reporting bias.
Assessment Process: 1) Independent Review: Two reviewers evaluated study design rigor, using adapted QUADAS-2 criteria for diabetes risk assessment. 2) Study Categorization: Included 7 cross-sectional, 6 cohort, and 1 case-control study. 3) Bias Judgment: Each study was rated as low, high, or unclear risk in QUADAS-2 domains. 4) Comparison with Previous Reviews: Unlike earlier findings [9][18], with 87.8% high risk of bias, our review found all 14 studies had low risk of bias, highlighting their methodological robustness. All studies were deemed low risk of bias and included in the final analysis (Table 2 in Appendix C).
RESULTS
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
Descriptive Overview of the Included Studies
This review includes 14 studies conducted across 11 countries, spanning the years 2009 to 2018. These studies were drawn from five databases and nine community-based settings, providing a diverse geographic and methodological landscape. The study designs included one case-control study [40], seven cross-sectional studies [41–47], and six cohort studies [18][31][48–51]. Participants were adults aged 18 and older who were identified as being at high risk for diabetes or prediabetes.
Summary of the diabetes risk screening tools
The types of diabetes risk assessment tools varied, with the following tools and models being assessed (Table 1):
1) Risk Screening Tools: Thai-RISK, CANRISK (3 studies), FINDRISC (3 studies), ADA, IDRS, UDD, and Filipino risk scores.
2) Comparative Models: OGTT, CANRISK Original, FBG, ADA, CDA, FPG, and A1C were used to benchmark and compare the efficacy of these risk tools.
3) The "gold standard" criteria or diagnostic cut-off points applied in these studies included CANRISK Original, ADA, CDA, FPG, OGTT, and A1C, allowing for standardized evaluation of risk assessment performance.
Table 1. Characteristics of the included studies (according to PICOs criteria)
|
First Authors |
Countries |
Study Design |
Participants (P) |
Index Test (I) Name of Tool |
Goal Standard / Cut Point (I) |
Timing of Study (C) |
Setting or Area (C) |
|
Agarwal G (2019) [40] |
Philippines |
Case-control |
Adults ≥40/≤40 yrs with impaired glucose |
CANRISK ≥33, FINDRISC ≥15, ADA ≥3, IDRS ≥60, UDD ≥14, Filipino ≥21 |
FPG≥100 mg/dL (5.6 mmol/L) per ADA |
2015–2016 |
Zamboanga City Health Center (PHAC) |
|
Aekplakorn W (2015) [41] |
Thailand |
Cross-sectional |
Adults 35–65 yrs with 1 or more of 6 common T2DM risk, CVD risk factors |
Thai Diabetes Risk Score, 6 risk factors |
FPG ≥100 mg/dL (5.6 mmol/L); OGTT ≥140 mg/dL (7.8 mmol/L) per ADA |
2013 |
68 primary care centers across all regions |
|
Bahijri S (2020) [42] |
Saudi Arabia |
Cross-sectional |
Adults 20–81 yrs with impaired glucose and T2DM risk |
SADRISC (≥15), FINDRISC |
FPG ≥100 mg/dL (5.6 mmol/L); OGTT (50 g, 1h-PG) 140–200 mg/dL; ADA criteria |
2016–2017 |
Public Health Care Centers |
|
Jiang Y (2017) [43] |
Canada |
Cross-sectional |
Adults 30 yrs and over with T2DM risk |
CANRISK ≥32 |
FPG >100 mg/dL (5.6 mmol/L); OGTT ≥140 mg/dL (7.8 mmol/L) per WHO |
2013 (2 months) |
Inuit communities, Nunavut |
|
Memish ZA (2015) [44] |
Saudi Arabia |
Cross-sectional |
Adults ≥20–45 yrs, non-pregnant |
SADRISC |
FPG ≥100 mg/dL (5.6 mmol/L); OGTT ≥140 mg/dL (7.8 mmol/L) per ADA |
2009 |
PHCCs Urban & Rural |
|
Rowan CP (2014) [45] |
Canada, Toronto |
Cross-sectional |
Adults ≥18 yrs with high T2DM risk |
CANRISK, FINDRISC |
HbA1c ≥5.7% (ADA), HbA1c ≥6.0% (CDA), HbA1c ≥6.5% |
2013 |
Community-based |
|
Srugo SA (2020) [46] |
Canada |
Cross-sectional |
Adults 18–39 yrs / Adults ≥40 yrs |
CANRISK ≥22 |
HbA1c ≥5.7% (ADA), HbA1c ≥6.0% (CDA), HbA1c ≥6.5% |
2018 |
Community-based |
|
Vanderwood KK (2015) [47] |
USA |
Cross-sectional |
Adults 40–75 yrs with high T2DM risk |
ADA risk test ≥9, CANRISK ≥19 |
OGTT (2h-PG) ≥140 mg/dL (7.8 mmol/L); ADA criteria |
2014 |
Worksites and community centers |
|
Risøy AJ (2018) [50] |
Norway |
Longitudinal study |
Adults ≥18 yrs with high T2DM risk |
FINDRISC, DM-UK |
HbA1c ≥5.7% (ADA) |
2016 (2 months) |
Community pharmacies |
|
Schmidt MI (2019) [51] |
Brazil |
Longitudinal study |
Adults 35–74 yrs (civil servants) |
Risk - Self-reported |
FPG ≥100 mg/dL (5.6 mmol/L) per ADA |
2008–2010 |
Healthcare centers |
|
Bethel MA (2013) [31] |
Asia, Europe, Latin America |
NAVIGATOR trial (Cohort, 5 years follow-up) |
Adults 45–70 yrs with impaired glucose |
Novel Risk Models A-E |
FPG ≥100 mg/dL (5.6 mmol/L); OGTT ≥140 mg/dL (7.8 mmol/L) |
Not stated |
Multinational |
|
Hippisley-Cox J (2017) [48] |
England |
Prospective Cohort study (3.9 years follow-up) |
Adults 25–84 yrs, non-diabetes |
QDiabetes models A-C |
FPG per ADA; HbA1c ≥5.7% |
2016 |
GP Practices (QResearch) |
|
Kaneko K (2020) [49] |
Japan |
Cohort study |
Adults 40–64 yrs with Mets and impaired glucose and T2DM risk |
IFG, MetS combinations |
FPG ≥100 mg/dL (5.6 mmol/L) |
2008–2018 |
Health centers |
|
Xu S (2021) [18] |
China |
Cohort study |
Adults with IGT from ACE study |
BASIC, EXTENDED, FULL models |
FPG ≥100 mg/dL (5.6 mmol/L); 2h-OGTT ≥140 mg/dL (7.8 mmol/L); HbA1c ≥5.7% |
Not stated |
ACE Study and LUSHOU cohort |
Note: FBG: Fasting Blood Glucose; OGTT: Oral Glucose Tolerance Test; HbA1c: Hemoglobin A1c; THAIRISK: Thai Diabetes Risk Score; CDA: Canadian Diabetes Association; CANRISK: Canadian Diabetes Risk Score; FINDRISC: Finnish Diabetes Risk Score; ADA RISK: American Diabetes Association Risk Score; IDRS: Indian Diabetes Risk Score; UDDM: Diabetes Risk Tools for Indonesia; Filipino: Diabetes Risk Tools for the Philippines; SADRISC: Saudi Arabia Diabetes Risk Tool; and UK Diabetes Risk.
Development of Diabetes Risk screening Tools
Developing reliable diabetes risk assessment tools is essential for early detection and diagnosis in adults with high-risk impaired glucose, intermediate hyperglycemia, or prediabetes. Low-cost, simple tools such as paper-based risk questionnaires or anthropometric measurements allow for easier identification of at-risk individuals who may benefit from lifestyle interventions. Common tools for assessing prediabetes or diabetes risk include the CANRISK, FINDRISC, and ADA Risk Questionnaires. The development of these tools generally falls into three main categories:
Non-invasive risk score assessments: These tools estimate diabetes risk based on non-invasive parameters, such as BMI, age, and lifestyle factors [42–44][47].
Index tests for diabetes risk scores: These studies investigate the utility of diabetes risk scores as diagnostic tools [40][41][45][46][50].
Predictive models for incident diabetes: These models focus on predicting the future development of diabetes in individuals identified as at risk [18][51].
In summary, this review outlines three key findings regarding diabetes risk screening tools (Table 2):
- Development methods: Approaches to creating and refining diabetes risk assessment tools.
- Detection capabilities: Effectiveness of the tools in identifying prediabetes or diabetes based on sensitivity, specificity, accuracy, and feasibility.
- Progression and intervention: Factors that influence the reversal of prediabetes risk and the likelihood of progressing to diabetes over time, with an estimated incidence of 15.9% over five years.
These findings highlight the need for tailored, effective risk assessment tools that are both accessible and accurate, enabling early intervention in at-risk populations.
Table 2. Diagnostic performance and cut-off scores of diabetes risk screening tools (9 studies)
|
Study (citation) |
Screening tool |
Cut-off score (risk threshold) |
Outcome predicted |
Reference standard & cut-point |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
|
Agarwal G (2019) [40] |
CANRISK |
≥33 |
Prediabetes or undiagnosed diabetes |
FPG ≥100 mg/dL (5.6 mmol/L) (ADA) |
70 |
67 |
35 |
|
FINDRISC |
≥15 |
78 |
67 |
13 |
|||
|
ADA RISK TEST |
≥3 |
79 |
67 |
10 |
|||
|
IDRS |
≥60 |
83 |
60 |
17 |
|||
|
UDDM |
≥14 |
- |
- |
- |
|||
|
Filipino Risk Score |
≥21 |
- |
- |
- |
|||
|
Aekplakorn W. (2015) [41] |
Thai Diabetes Risk Score |
≥6-FPG |
Prediabetes (screen positive) |
FPG ≥100 mg/dL (5.6 mmol/L) or 2-h OGTT ≥140 mg/dL (7.8 mmol/L) (ADA) |
53.7 |
100 |
100 |
|
≥6-OGTT |
81.1 |
100 |
100 |
||||
|
Bahijri S (2020) [42] |
SADRISC |
≥4 |
Prediabetes or diabetes |
FPG ≥100 mg/dL (5.6 mmol/L); OGTT (50 g 1-h PG) 140–200 mg/dL (ADA) |
87 |
45 |
33 |
|
≥5 |
72 |
66 |
39 |
||||
|
≥6 |
69 |
69 |
40 |
||||
|
≥7 |
53 |
83 |
49 |
||||
|
Jiang Y (2017) [43] |
CANRISK |
≥21 |
Prediabetes (WHO criteria) |
FPG >100 mg/dL (5.6 mmol/L); 2-h OGTT ≥140 mg/dL (7.8 mmol/L) (WHO) |
85.1 |
31.4 |
26.0 |
|
≥29 |
65.7 |
55.5 |
29.5 |
||||
|
≥32 |
61.2 |
65.7 |
33.6 |
||||
|
≥33 |
61.2 |
66.5 |
34.2 |
||||
|
Memish ZA (2015) [44] |
SADRISC |
≥5 |
Prediabetes or diabetes |
FPG ≥100 mg/dL (5.6 mmol/L); OGTT ≥140 mg/dL (7.8 mmol/L) (ADA) |
71.2 |
54 |
- |
|
Rowan CP (2014) [45] |
FINDRISC |
≥6.5 |
Prediabetes or diabetes |
HbA1c ≥5.7% (ADA) / ≥6.0% (CDA) / ≥6.5% (diabetes) |
85.3 |
43.5 |
- |
|
Srugo SA (2020) [46] |
New CANRISK |
≥22 |
Prediabetes or diabetes |
HbA1c ≥5.7% (ADA)/ other thresholds per study |
78.8 |
54.0 |
- |
|
Original CANRISK |
77.1 |
55.0 |
- |
||||
|
Vanderwood KK (2015) [47] |
ADA Risk Test |
≥9 |
Prediabetes or diabetes |
2-h OGTT ≥140mg/dL (7.8 mmol/L) and ADA/CDA definitions |
68.9–98.5 |
44.5 |
- |
|
Schmidt MI (2019) [51] |
QDiabetes-style approach |
Model-specific (10-yr risk ≥10%) |
Incident diabetes / 10-yr risk |
FPG ≥100 mg/dL (5.6 mmol/L) (ADA) as diagnostic reference in the study |
67.7 |
77.9 |
- |
Note: Outcome Predicted — whether the screening tool correctly identifies individuals with prediabetes and/or undiagnosed diabetes as defined by the reference standard in each study (FPG, OGTT, or HbA1c based on ADA/WHO criteria). Cut-off scores are as reported in the original studies.
ADA RISK — American Diabetes Association Risk Score; CANRISK — Canadian Diabetes Risk Score; CDA — Canadian Diabetes Association; FBG — Fasting Blood Glucose; Filipino — Diabetes Risk Tools for the Philippines; FINDRISC — Finnish Diabetes Risk Score; IDRS — Indian Diabetes Risk Score; OGTT — Oral Glucose Tolerance Test; PG — Plasma Glucose; PPV — Positive Predictive Value; SADRISC — Saudi Arabia Diabetes Risk Tool; UDDM — Diabetes Risk Tools for Indonesia; WHO — World Health Organization.
Progression to Diabetes
Across 14 studies, 8.69 million participants were analyzed, with 53% female and a mean age of 38.4 years (SD=7.7). Risk assessments included 5 to 15 factors (median: 11), categorized as follows (Table 3):
- Socioeconomic:age, sex, education, marital status, and occupation.
- Anthropometric:BMI, weight, waist circumference, and waist-to-hip and waist-to-height ratios.
- Biomarkers:blood pressure, lipid profiles (total cholesterol, HDL, TG, LDL), hypertension history, gestational diabetes, and family history of diabetes.
- Lifestyle:Smoking, physical activity, and sleep duration.
Among 6.49 million (74.7%) who completed risk assessments: 3.1% (198,968) were identified as high-risk for prediabetes, 2.8% (180,639) were diagnosed with diabetes during follow-up. Among diagnosed cases, the average diabetes duration was 5.5 years.
Table 3. Baseline characteristics of the included studies
|
First Author (Year) |
Sample Size (Case/Control) |
Mean Age (SD) |
Sex (M/F, %) |
Number of Risk Factors |
High Risk Identified (%) |
Complete Risk Assessment (%) |
High Risk of Pre-DM (%) |
Diagnosed with DM (%) |
|
Agarwal G (2019) [40] |
200 (50/150) |
56 (11.5) |
M: 23.5, F: 76.5 |
9 |
NA |
100 |
8 |
6 |
|
Aekplakorn W (2015) [41] |
6,884 |
50.5 (6.9) |
M: 23.6, F: 76.4 |
6 |
38.8 |
88 |
38.8 |
13.4 |
|
Bahijri S (2020) [42] |
1,477 |
32 (11.5) |
M: 53.6, F: 47.4 |
11 |
22.2 |
23 |
17.5 |
4.7 |
|
Jiang Y (2017) [43] |
303 |
<45 (50%) |
M: 34.4, F: 65.6 |
12 |
6.7 |
100 |
18 |
4 |
|
Memish ZA (2015) [44] |
1,485 |
50–59 (64%) |
M: 62, F: 38 |
7 |
49.2 |
96.6 |
49.2 |
16 |
|
Rowan CP (2014) [45] |
691 |
<40 (32.3%) |
M: 29, F: 71 |
7 |
ADA: 79.7, CDA: 75 |
85.2 |
ADA: 79.7, CDA: 75 |
61.7 |
|
Srugo SA (2020) [46] |
3,334 |
28.5 (NA) |
M: 37.6, F: 62.4 |
13 |
NA |
100 |
5.8 |
1.5 |
|
Vanderwood KK (2015) [47] |
364 |
55.8 (12.5) |
M: 36, F: 64 |
7 |
89 |
86 |
55 |
19.4 |
|
Risøy AJ (2018) [50] |
211 |
<45 (43%) |
M: 40, F: 60 |
8 |
6.6 |
100 |
5.4 |
1.4 |
|
Schmidt MI (2019) [51] |
15,105 |
45–54 (32%) |
M: 45.5, F: 54.5 |
5 |
79 |
74.1 |
59 |
2% (person-year) |
|
Bethel MA (2013) [31] |
9,306 |
63.8 (6.8) |
M: 49, F: 51 |
15 |
49 |
100 |
35 |
35 |
|
Hippisley-Cox J (2017) [48] |
8,640,363 |
44.9 (15.2) |
M: 49.6, F: 50.4 |
12 |
NA |
96.9 |
28.2 |
19.1 |
|
Kaneko K (2020) [49] |
8,989 |
50 (NA) |
M: 82.7, F: 17.3 |
11 |
43.3 |
46 |
18.8 |
5.8 |
|
Xu S (2021) [18] |
3,250 |
63 (NA) |
M: 72.4, F: 27.6 |
15 |
NA |
96 |
15.8 |
21.1 |
|
14 authors |
200 to 8.6 million participants, reflecting diverse population sizes. |
from young (28.5 years) to older (63.8 years). |
Male-to-female ratios were mostly balanced, with a few studies having male-dominated cohorts (e.g., Kaneko K: 82.7% male). |
Risk factors assessed ranged from 5 to 15, showing different screening approaches. |
High-risk identification rates varied widely (6.6% to 79.7%). |
Most studies achieved over 85% completion rates for assessments. |
The prevalence of pre-diabetes among high-risk individuals ranged from 5.4% to 55%. |
Diabetes diagnosis rates ranged from 1.4% to 35%, depending on population and study design. |
Note: NA: Not applicable, NS: NOT State, FBG: Fasting Blood Glucose, OGTT: Oral Glucose Tolerance Test, A1C: Hemoglobin A1C, ECG: electrocardiogram, THAIRISK: Thai Diabetes Risk Score, CDA: Canadian Diabetes Association, CANDRISK: Canadian Diabetes Risk Score FINDRISC ; Finnish Diabetes Risk Score, ADA RISK: America Diabetes Association Risk Score, IDRS; Indian Diabetes Risk Score, UDDM; Diabetes Risk tools for Indonesia, Filipino; Diabetes Risk tools for Philippine, SADRISC: Saudi Arabia diabetes risk tool, UK-diabetes risk
Summary of risk factors, score ranges, and accuracy of the screening tools
Key risk factors in diabetes screening tools include age, BMI, history of diabetes (HxDM), hypertension (HT), and waist circumference (WC). Some tools incorporate additional factors (Table 4):
- Sex:Thai-RISK, CANRISK-9, Filipino Risk Score
- Physical activity:Included in most tools except Thai-RISK
- Diet:CANRISK-9, FINDRISC-8, SADRISC-10, UK-D-10
- Gestational diabetes (GDM):ADA-7, SADRISC-10, UK-D-10, Filipino Risk Score
- Ethnicity: CANRISK-9, UK-D-10, SADRISC-10
- Smoking:CANRISK-9, SADRISC-10, Filipino Risk Score
- Excluded factors across all tools:lipid levels (HDL, LDL), cardiovascular diseases (CVD), and corticosteroid use.
Tool Performance:
- Sensitivity:70–90% (higher in Thai-RISK, CANRISK-9, FINDRISC-8, and UK-D-10).
- Specificity:45–80% (higher in CANRISK-9, FINDRISC-8).
Risk Stratification:
- Low risk:Below tool-specific cut-off.
- Moderate risk:Intermediate range.
- High risk:Exceeds high-risk cut-off, indicating a greater likelihood of diabetes.
The tools that had more risk factors had lower % of people identified at high risk of pre-DM, suggesting that adding more risk factors doesn't improve prediction.
Table 4. Risk factors, Score Range, Cut-off score and Sensitivity (%) Specificity (%) of the screening tools
|
Risk factors |
Thai-RISK |
CANRISK-9 |
FINDRISC-8 |
ADA-7 |
UK-D-10 |
SADRISC-10 |
IDRS-4 |
UDD-7 |
Filipino risk scors-9 |
|
1) Age |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
2) Sex |
+ |
+ |
- |
- |
- |
- |
- |
- |
+ |
|
3) BMI |
+ |
+ |
- |
+ |
+ |
- |
- |
+ |
+ |
|
4) Hx of DM |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
5) HT (Hypertension) |
+ |
+ |
+ |
+ |
+ |
+ |
- |
+ |
+ |
|
6) WC (Waist Circumference) |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
7) Impaired Glucose |
+ |
- |
+ |
- |
+ |
+ |
- |
+ |
- |
|
8) Physical Activity |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
9) Diet |
- |
+ |
+ |
- |
+ |
+ |
- |
- |
- |
|
10) GDM (woman) |
- |
- |
- |
+ |
+ |
+ |
- |
+ |
+ |
|
11) Ethnicity |
- |
+ |
- |
- |
+ |
+ |
- |
- |
- |
|
12) Smoking |
- |
+ |
- |
- |
- |
+ |
- |
- |
+ |
|
13) Lipids (HDL, LDL) |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
14) CVD |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
15) Drug (Depression, schizophrenia, corticosteroids) |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
N of risk factors |
7 |
9 |
8 |
7 |
10 |
10 |
4 |
7 |
9 |
|
Sensitivity (%) |
80–90% |
75–85% |
78–88% |
70–85% |
81% |
~80% |
75–85% |
~80% |
78–88% |
|
Specificity (%) |
60–75% |
70–80% |
72–80% |
65–75% |
45% |
~70% |
65–75% |
~75% |
70–80% |
|
Range of score |
0–17 |
0–100 |
0–26 |
0–10 |
0–47 |
0–15 |
0–100 |
0–24 |
0–25 |
|
Cut-off score |
≥6 |
≥ 33 |
≥15 |
≥3 |
≥16 |
≥5 |
≥60 |
≥9 |
≥9 |
|
Low risk score |
<6 |
<21 |
<7 |
<5 |
≤16 |
≤5 |
<30 |
≤9 |
≤9 |
|
Moderate risk score |
6–8 |
21–32 |
7–11 |
- |
17–24 |
6–9 |
30–50 |
10–14 |
10–14 |
|
High risk score |
≥9 |
>32 |
12–20 |
≥5 |
≥25 |
≥10 |
≥60 |
≥15 |
≥15 |
|
Very high risk score |
- |
- |
≥ 21 |
- |
- |
- |
- |
- |
- |
Note: (+): Yes; (-): No
KEY FINDINGS AND IMPLICATIONS
The 14 studies included a range of screening tools with different cutoffs and several different reference (i.e. gold) standards, with limited repetition of these combinations of tools, cutoffs and reference standards across the studies (Table 1 and Table 2). This makes it difficult to determine how generalisable the findings from individual studies would be. Overall, 26 screening tools were assessed across the 14 studies.
Across all studies and all combinations of the screening tools and their corresponding reference standards, sensitivity of the tools for detecting people with prediabetes or those at high risk of developing diabetes ranged from 54% to 98% whilst specificity ranged from 31.5% to 100% and the PPV ranged from 10% to 100% (Table 2). Thus, none of the tools studied optimised both sensitivity and specificity and, in all cases, the PPV (i.e. the probability that someone with a positive screening test result would actually have prediabetes or a high risk of developing diabetes) was relatively low. The screening tools with the highest sensitivity for identifying people with prediabetes or a high risk of developing type 2 diabetes were ADA Risk Tes with a ≥9 cutoff, FINDRISC ≥15 cutoff and IDRS with a ≥60 cutoff (94% and 92% sensitivity respectively) but in the corresponding specificity was relatively low (43%, 45% and 67% respectively) (Table 2).
There is a strong need for more sensitive and specific tools to identify prediabetes effectively. While CANRISK and FINDRISC show potential for early detection, greater internal validation is essential to ensure their reliability across populations. Strengthening validation efforts can enhance screening accuracy, guiding health providers and policymakers in developing targeted preventive strategies for high-risk groups.
This review analyzed one case-control study, seven cross-sectional studies, and six cohort studies focused on tool development and validation.
DISCUSSION
This scoping review provides a comprehensive synthesis of diabetes risk screening tools for identifying adults at risk of prediabetes or type 2 diabetes mellitus (T2DM), as follow each objective of the study;
Characteristics of Diabetes Risk Screening Tools
We identified 14 studies representing 26 risk models, including widely used tools such as CANRISK, FINDRISC, and ADA-Risk, with considerable variability in sensitivity, specificity, and applicability across populations [42–44][47]. The majority use indirect predictors such as BMI, family history of diabetes, age, waist circumference and physical activity [44][49]. Similarly, Rowan et al. [45]. Studies show that the standard CANRISK questionnaire, with a cut-off score of 33 points, achieves good accuracy, while a lower cut-off of 21 points significantly increases sensitivity [52]. However, adjusting BMI and waist circumference cut-off points for ethnicity did not enhance predictive accuracy. A risk score based on factors such as sex, age, waist circumference, hyperglycemia history, and family diabetes history, with scores ranging from 0 to 15, was deemed effective for assessing prediabetes or diabetes risk [40][42]. Women with impaired fasting glucose (IFG) are often underdiagnosed, and using OGTT may improve prediabetes detection, especially among women aged 45 and older [41]. Between simplicity and utility are some of the tools (eg, Thai-RISK and CANRISK) that were developed with restricted external validation so their extrapolation outside the study population should be with caution [40][41][45][46][50]. A model combining FBG and OGTT (C=0.70) proved effective for diabetes risk assessment [31]. However, models based on IFG (WHO criteria) had lower sensitivity (67.7%) and specificity (77.9%) than expected.
Effectiveness and Feasibility of Diabetes Risk Assessment Tools
The effectiveness of various tools in predicting T2DM was assessed through their ability to classify individuals based on risk factors like impaired glucose tolerance (IGT) and impaired fasting glucose (IFG). For instance, the CANRISK tool has demonstrated success in identifying impaired glucose in Canada’s First Nations and Métis communities, as well as in Saudi Arabia [40][41][45][46][50]. Jieng’s study suggested that OGTT was superior to FPG for risk prediction [43], and Norway’s community pharmacies effectively utilized HbA1c testing to identify undiagnosed T2DM cases. The QDiabetes-2018 algorithm further showed strong predictive capabilities for 10-year risk of T2DM, confirming its feasibility for use in community screening [41][51]. Screening through FINDRISC in pharmacy settings proved feasible, highlighting the utility of accessible locations for reaching at-risk populations. Suggested model cut points include A-Q Diabetes (no FBG or A1c), B-FBG, and C-A1C [48]. For 10-year diabetes risk prediction, Model B_FBG provided the best performance, effectively identifying individuals needing intervention or more intensive follow-up [48]. Other studies found the risk score approach feasible and effective for assessing diabetes risk in community and pharmacy settings, allowing both pharmacists and participants to engage in the screening process [44][50]. Simple, cost-effective questionnaires are valuable for raising awareness and assessing type 2 diabetes risk [49]. Aside from the SADRISC tool and ADA Risk Test, which were each included in two studies, all other diabetes screening tools were only included in one study and so their sensitivity, specificity and PPV in a wider range of settings is unknown. Due to the different tools and reference standards used across the studies a quantitative meta-analysis of the studies is not feasible. Our risk of bias assessment using QUADAS2 suggested that the studies were broadly similar in their risk of bias, but the studies differed in a range of factors, including the country, study design, age range, and risk factors of the populations (Table 1). These factors likely contribute to the observed heterogeneity of the sensitivity, specificity and PPV results but are difficult to test for as explanatory variables in this evidence base due to the limited occurrence of each set of variables across the studies.
Progression from Prediabetes to Diabetes
Cohort studies primarily examined the progression of individuals with prediabetes to diabetes, emphasizing IFG and Metabolic syndrome (MetS) as significant risk factors [18][31][51]. Kaneko et al. found that IFG held a higher population-attributable fraction (PAF) than MetS in predicting T2DM incidence among middle-aged Japanese participants [25], and the coexistence of IFG and MetS showed the highest risk [9]. This finding suggests that IFG could be a valuable marker for diabetes risk, especially when used in combination with MetS criteria. In Saudi Arabia and Algeria, basic assessment tools have effectively evaluated diabetes risk in the population. Across studies, socioeconomic factors such as age, education, and marital status, as well as biomarkers like blood pressure and lipid levels, were found to significantly impact diabetes progression [18][31][51].
In a five-year period, 15.9% of participants with impaired glucose tolerance (IGT) or coronary heart disease (CHD) progressed to diabetes, underscoring the importance of targeted interventions. A risk prediction model utilizing clinical variables readily available in routine practice can help estimate diabetes risk in specific populations, such as Chinese individuals with CHD or IGT. Despite some studies having small sample sizes, the general recommendation is for settings to raise diabetes risk awareness for individuals scoring 9 or higher on the ADA risk test [18][47]. If the intention is primarily to identify people with prediabetes or at high risk of developing type 2 diabetes then the relatively low specificity and PPV values may not be a concern, provided that there are no negative issues associated with false positive results (such as the costs of testing or of the management of patients who receive a negative diagnosis).
CONCLUSIONS
This review mapped the field of diabetes risk screening tools and emphasized the importance of more systematic validation, particularly in different populations. A range of different screening tools has been tested that could identify people with prediabetes or a high risk of developing type 2 diabetes. However, where sufficient evidence was available to compare tools across studies the performance of these tools was inconsistent. Several tools have only been investigated in single studies, with uncertainty around their wider generalisability. Clinicians or researchers wishing to screen people for prediabetes or a high risk of developing type 2 diabetes using any of these tools should be aware of their potential limitations.
Limitations: The limitations of this review are that it only covers English and Thai literature; possible publication bias due to the fact that grey literature is not included; and that no meta-analysis was conducted. Although this is to be expected in scoping reviews, the reporting of pooled performance ranges in our tables serves to narrow the knowledge gap and achieve higher interpretability.
Strengths: Diverse Geographic Coverage: Findings are generalizable across different populations. Comprehensive Tool Review: Analyzing 14 screening tools provides a broad comparative perspective. Quality Assessment: QUADAS-2 ensures structured evaluation, enhancing reliability. Clinical Relevance: Identifies practical, non-invasive risk factors (e.g., age, BMI) for easy application in healthcare settings.
ADDITIONAL INFORMATION
Source of funding. This research received no external funding.
Conflict of interests. Authors declare no explicit and potential conflicts of interests associated with the publication of this article.
Authors involvement. P.W.: Conceptualization, methodology, investigation, resources, data curation; P.W., S.D.; validation, formal analysis, P.W.; S.D., and TTN.; writing-original; All authors read and final proof the manuscript.
Acknowledgements. We thank for our information specialists and all advisory board of the community medicine division, Thammasat University, for their help in developing the search strategy and for selecting databases. Special thanks to Dr. Geoff K. Frampton, the Principal Research fellow from the School of Medicine, University of Southampton, UK. and Associate Prof. Dr. Omid Dadras, Queensland Ambulance Services, Queensland Government Department of Health, Brisbane, Australia.
References
1. Aekplakorn, W., Bunnag, P., Woodward, M., Sritara, P., Cheepudomwit, S., Yamwong, S., Yipintsoi, T., & Rajatanavin, R. (2006). A risk score for predicting incident diabetes in the Thai population. Diabetes Care, 29(8), 1872-1877. https://doi.org/10.2337/dc05-2141
2. Aekplakorn, W., Tantayotai, V., Numsangkul, S., Sripho, W., Tatsato, N., Burapasiriwat, T., Pipatsart, R., Sansom, P., Luckanajantachote, P., Chawarokorn, P., Thanonghan, A., Lakhamkaew, W., Mungkung, A., Boonkean, R., Chantapoon, C., Kungsri, M., Luanseng, K., & Chaiyajit, K. (2015). Detecting Prediabetes and Diabetes: Agreement between Fasting Plasma Glucose and Oral Glucose Tolerance Test in Thai Adults. Journal of Diabetes Research, 2015, 396505. https://doi.org/10.1155/2015/396505
3. Agarwal, G., Guingona, M. M., Gaber, J., Angeles, R., Rao, S., & Cristobal, F. (2019). Choosing the most appropriate existing type 2 diabetes risk assessment tool for use in the Philippines: a case-control study with an urban Filipino population. BMC Public Health, 19(1), 1169. https://doi.org/10.1186/s12889-019-7402-0
4. Agarwal, G., Jiang, Y., Rogers Van Katwyk, S., Lemieux, C., Orpana, H., Mao, Y., Hanley, B., Davis, K., Leuschen, L., & Morrison, H. (2018). Effectiveness of the CANRISK tool in the identification of dysglycemia in First Nations and Métis in Canada. Health Promot Chronic Dis Prev Can, 38(2), 55-63. https://doi.org/10.24095/hpcdp.38.2.02 (Efficacité de l’outil CANRISK pour détecter la dysglycémie chez les membres des Premières Nations et les Métis au Canada.)
5. American Diabetes, A. (2020). 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care, 44(Supplement_1), S15-S33. https://doi.org/10.2337/dc21-S002
6. American Diabetes Association. (2019a). 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care, 43(Supplement_1), S14-S31. https://doi.org/10.2337/dc20-S002
7. American Diabetes Association. (2019b). 3. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care, 43(Supplement_1), S32-S36. https://doi.org/10.2337/dc20-S003
8. American Diabetes Association. (2022). Standards of Medical Care in Diabetes—2022 Abridged for Primary Care Providers. Clinical Diabetes, 40(1), 10-38. https://doi.org/10.2337/cd22-as01
9. Amisi, C. A. (2022). Markers of insulin resistance in Polycystic ovary syndrome women: An update. World Journal of Diabetes, 13(3), 129.
10. Bahijri, S., Al-Raddadi, R., Ajabnoor, G., Jambi, H., Al Ahmadi, J., Borai, A., Barengo, N. C., & Tuomilehto, J. (2020). Dysglycemia risk score in Saudi Arabia: A tool to identify people at high future risk of developing type 2 diabetes. J Diabetes Investig, 11(4), 844-855. https://doi.org/10.1111/jdi.13213
11. Baker, P., & El-Osta, A. (2019). Who self‐cares wins: a global perspective on men and self-care.
12. Barber, S. R., Davies, M. J., Khunti, K., & Gray, L. J. (2014). Risk assessment tools for detecting those with pre-diabetes: a systematic review. Diabetes Res Clin Pract, 105(1), 1-13. https://doi.org/10.1016/j.diabres.2014.03.007
13. Bethel, M. A., Chacra, A. R., Deedwania, P., Fulcher, G. R., Holman, R. R., Jenssen, T., Kahn, S. E., Levitt, N. S., McMurray, J. J., Califf, R. M., Raptis, S. A., Thomas, L., Sun, J. L., & Haffner, S. M. (2013). A novel risk classification paradigm for patients with impaired glucose tolerance and high cardiovascular risk. Am J Cardiol, 112(2), 231-237. https://doi.org/10.1016/j.amjcard.2013.03.019
14. Beulens, J., Rutters, F., Rydén, L., Schnell, O., Mellbin, L., Hart, H. E., & Vos, R. C. (2019). Risk and management of pre-diabetes. Eur J Prev Cardiol, 26(2_suppl), 47-54. https://doi.org/10.1177/2047487319880041
15. Blonde, L., Umpierrez, G. E., McGill, J. B., Reddy, S. S., Berga, S. L., Bush, M., Chandrasekaran, S., DeFronzo, R. A., Einhorn, D., & Galindo, R. (2022). American Association of Clinical Endocrinology Clinical Practice Guideline: Developing a Diabetes Mellitus Comprehensive Care Plan—2022 Update. Endocrine Practice.
16. Booth, F. W., Roberts, C. K., & Laye, M. J. (2012). Lack of exercise is a major cause of chronic diseases. Compr Physiol, 2(2), 1143-1211. https://doi.org/10.1002/cphy.c110025
17. Cho, N. H., Shaw, J. E., Karuranga, S., Huang, Y., da Rocha Fernandes, J. D., Ohlrogge, A. W., & Malanda, B. (2018). IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract, 138, 271-281. https://doi.org/10.1016/j.diabres.2018.02.023
18. Dhippayom, T., Chaiyakunapruk, N., & Krass, I. (2014). How diabetes risk assessment tools are implemented in practice: A systematic review. Diabetes Research and Clinical Practice, 104(3), 329-342. https://doi.org/https://doi.org/10.1016/j.diabres.2014.01.008
19. Doddamani, P., Ramanathan, N., Swetha, N. K., & Suma, M. N. (2021). Comparative Assessment of ADA, IDRS, and FINDRISC in Predicting Prediabetes and Diabetes Mellitus in South Indian Population. J Lab Physicians, 13(1), 36-43. https://doi.org/10.1055/s-0041-1727557
20. Dreher, M. L. (2018). Dietary patterns and whole plant foods in aging and disease. Springer.
21. Garay, J., Camacho, P. A., Lopez-Lopez, J., Alvernia, J., Garcia, M., Cohen, D. D., Calderon, C., & Lopez-Jaramillo, P. (2019). Survey of knowledge for diagnosing and managing prediabetes in Latin-America: cross-sectional study. Diabetol Metab Syndr, 11, 102. https://doi.org/10.1186/s13098-019-0500-4
22. Guess, N. D., Caengprasath, N., Dornhorst, A., & Frost, G. S. (2015). Adherence to NICE guidelines on diabetes prevention in the UK: Effect on patient knowledge and perceived risk. Prim Care Diabetes, 9(6), 407-411. https://doi.org/10.1016/j.pcd.2015.04.005
23. Hippisley-Cox, J., & Coupland, C. (2017). Development and validation of QDiabetes-2018 risk prediction algorithm to estimate future risk of type 2 diabetes: cohort study. BMJ, 359, j5019. https://doi.org/10.1136/bmj.j5019
24. IDF. (2021). IDF Diabetes Atlas 10th edition, https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf
25. Jellinger, P. S., Handelsman, Y., Rosenblit, P. D., Bloomgarden, Z. T., Fonseca, V. A., Garber, A. J., Grunberger, G., Guerin, C. K., Bell, D. S. H., Mechanick, J. I., Pessah-Pollack, R., Wyne, K., Smith, D., Brinton, E. A., Fazio, S., & Davidson, M. (2017). American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract, 23(Suppl 2), 1-87. https://doi.org/10.4158/ep171764.Appgl
26. Jiang, Y., Rogers Van Katwyk, S., Mao, Y., Orpana, H., Argwal, G., de Groh, M., Skinner, M., Clarke, R., & Morrison, H. (2017). Assessment of dysglycemia risk in the Kitikmeot region of Nunavut: using the CANRISK tool. Health Promot Chronic Dis Prev Can, 37(4), 114-122. https://doi.org/10.24095/hpcdp.37.4.02 (Évaluation du risque de dysglycémie dans la région de Kitikmeot (Nunavut) au moyen de l’outil CANRISK.)
27. Kaneko, K., Yatsuya, H., Li, Y., Uemura, M., Chiang, C., Hirakawa, Y., Ota, A., Tamakoshi, K., & Aoyama, A. (2020). Risk and population attributable fraction of metabolic syndrome and impaired fasting glucose for the incidence of type 2 diabetes mellitus among middle-aged Japanese individuals: Aichi Worker's Cohort Study. J Diabetes Investig, 11(5), 1163-1169. https://doi.org/10.1111/jdi.13230
28. Lin, L., Wang, A., He, Y., Wang, W., Gao, Z., Tang, X., Yan, L., Wan, Q., Luo, Z., Qin, G., Chen, L., Mu, Y., & Dou, J. (2021). Effects of the hemoglobin glycation index on hyperglycemia diagnosis: Results from the REACTION study. Diabetes Research and Clinical Practice, 180, 109039. https://doi.org/https://doi.org/10.1016/j.diabres.2021.109039
29. Luo, H., Chen, Z., Bell, R., Rafferty, A. P., Gaskins Little, N. R., & Winterbauer, N. (2020). Health Literacy and Health Behaviors Among Adults With Prediabetes, 2016 Behavioral Risk Factor Surveillance System. Public Health Rep, 135(4), 492-500. https://doi.org/10.1177/0033354920927848
30. Mata-Cases, M., Artola, S., Escalada, J., Ezkurra-Loyola, P., Ferrer-García, J. C., Fornos, J. A., Girbés, J., & Rica, I. (2015). Consensus on the detection and management of prediabetes. Consensus and Clinical Guidelines Working Group of the Spanish Diabetes Society. Endocrinol Nutr, 62(3), e23-36. https://doi.org/10.1016/j.endonu.2014.10.008 (Consenso sobre la detección y el manejo de la prediabetes. Grupo de Trabajo de Consensos y Guías Clínicas de la Sociedad Española de Diabetes.)
31. McCullough, D., Harrison, T., Boddy, L. M., Enright, K. J., Amirabdollahian, F., Schmidt, M. A., Doenges, K., Quinn, K., Reisdorph, N., & Mazidi, M. (2022). The Effect of Dietary Carbohydrate and Fat Manipulation on the Metabolome and Markers of Glucose and Insulin Metabolism: A Randomised Parallel Trial. Nutrients, 14(18), 3691.
32. Memish, Z. A., Chang, J. L., Saeedi, M. Y., Al Hamid, M. A., Abid, O., & Ali, M. K. (2015). Screening for Type 2 Diabetes and Dysglycemia in Saudi Arabia: Development and Validation of Risk Scores. Diabetes Technol Ther, 17(10), 693-700. https://doi.org/10.1089/dia.2014.0267
33. Mirasol, R., Thai, A. C., Salahuddin, A. A., Tan, K., Deerochanawong, C., Mohamed, M., Saraswati, M. R., Sethi, B. K., Shah, S., Soetedjo, N. N., Suraamornkul, S., Tan, R., & Uddin, F. (2017). A Consensus of Key Opinion Leaders on the Management of Pre-diabetes in the Asia-Pacific Region. J ASEAN Fed Endocr Soc, 32(1), 6-12. https://doi.org/10.15605/jafes.032.01.02
34. Numpong S, T. Y., Sungkhabut W. (2020). A systematic literature review of the diagnosis of drug resistant tuberculosis using Xpert MTB/RIF assay and its potential impacts on treatment outcomes [Research article]. https://he01.tci-thaijo.org/index.php/DCJ/article/view/216821, 46(3), 303-312. https://doi.org/ https://doi.org/10.14456/dcj.2020.29
35. Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., . . . Moher, D. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ, 372, n71. https://doi.org/10.1136/bmj.n71
36. Qiu, L., Wang, W., Sa, R., & Liu, F. (2021). Prevalence and Risk Factors of Hypertension, Diabetes, and Dyslipidemia among Adults in Northwest China. Int J Hypertens, 2021, 5528007. https://doi.org/10.1155/2021/5528007
37. Rauh, S. P., Rutters, F., van der Heijden, A., Luimes, T., Alssema, M., Heymans, M. W., Magliano, D. J., Shaw, J. E., Beulens, J. W., & Dekker, J. M. (2018). External Validation of a Tool Predicting 7-Year Risk of Developing Cardiovascular Disease, Type 2 Diabetes or Chronic Kidney Disease. J Gen Intern Med, 33(2), 182-188. https://doi.org/10.1007/s11606-017-4231-7
38. Risøy, A. J., Kjome, R. L. S., Sandberg, S., & Sølvik, U. (2018). Risk assessment and HbA1c measurement in Norwegian community pharmacies to identify people with undiagnosed type 2 diabetes - A feasibility study. PLoS One, 13(2), e0191316. https://doi.org/10.1371/journal.pone.0191316
39. Rosedale, M., Strauss, S. M., Knight, C., & Malaspina, D. (2015). Awareness of Prediabetes and Diabetes among Persons with Clinical Depression. International Journal of Endocrinology, 2015, 839152. https://doi.org/10.1155/2015/839152
40. Rowan, C. P., Miadovnik, L. A., Riddell, M. C., Rotondi, M. A., Gledhill, N., & Jamnik, V. K. (2014). Identifying persons at risk for developing type 2 diabetes in a concentrated population of high risk ethnicities in Canada using a risk assessment questionnaire and point-of-care capillary blood HbA1c measurement. BMC Public Health, 14, 929. https://doi.org/10.1186/1471-2458-14-929
41. Schmidt, M. I., Bracco, P. A., Yudkin, J. S., Bensenor, I. M., Griep, R. H., Barreto, S. M., Castilhos, C. D., & Duncan, B. B. (2019). Intermediate hyperglycaemia to predict progression to type 2 diabetes (ELSA-Brasil): an occupational cohort study in Brazil. Lancet Diabetes Endocrinol, 7(4), 267-277. https://doi.org/10.1016/s2213-8587(19)30058-0
42. Srugo, S. A., Morrison, H. I., Villeneuve, P. J., de Groh, M., & Jiang, Y. (2020). Assessing Dysglycemia Risk Among Younger Adults: A Validation of the Canadian Diabetes Risk Questionnaire. Can J Diabetes, 44(5), 379-386.e373. https://doi.org/10.1016/j.jcjd.2019.11.002
43. Sun, H., Saeedi, P., Karuranga, S., Pinkepank, M., Ogurtsova, K., Duncan, B. B., Stein, C., Basit, A., Chan, J. C. N., Mbanya, J. C., Pavkov, M. E., Ramachandaran, A., Wild, S. H., James, S., Herman, W. H., Zhang, P., Bommer, C., Kuo, S., Boyko, E. J., & Magliano, D. J. (2022). IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract, 183, 109119. https://doi.org/10.1016/j.diabres.2021.109119
44. Vanderwood, K. K., Kramer, M. K., Miller, R. G., Arena, V. C., & Kriska, A. M. (2015). Evaluation of non-invasive screening measures to identify individuals with prediabetes. Diabetes Res Clin Pract, 107(1), 194-201. https://doi.org/10.1016/j.diabres.2014.06.003
45. Vanderwood, K. K., Kramer, M. K., Miller, R. G., Arena, V. C., & Kriska, A. M. (2015). Evaluation of non-invasive screening measures to identify individuals with prediabetes. Diabetes Research and Clinical Practice, 107(1), 194-201. https://doi.org/https://doi.org/10.1016/j.diabres.2014.06.003
46. Wade, R., Corbett, M., & Eastwood, A. (2013). Quality assessment of comparative diagnostic accuracy studies: our experience using a modified version of the QUADAS-2 tool. Res Synth Methods, 4(3), 280-286. https://doi.org/10.1002/jrsm.1080
47. Whiting, P., Rutjes, A., Westwood, M., Mallett, S., Deeks, J., Reitsma, J., Leeflang, M., Sterne, J., & Bossuyt, P. (2011). QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Annals of Internal Medicine, 155, 529-536. https://doi.org/10.1059/0003-4819-155-8-201110180-00009
48. Xu, S., Coleman, R. L., Wan, Q., Gu, Y., Meng, G., Song, K., Shi, Z., Xie, Q., Tuomilehto, J., Holman, R. R., Niu, K., & Tong, N. (2022). Risk prediction models for incident type 2 diabetes in Chinese people with intermediate hyperglycemia: a systematic literature review and external validation study. Cardiovasc Diabetol, 21(1), 182. https://doi.org/10.1186/s12933-022-01622-5
49. Xu, S., Scott, C. A. B., Coleman, R. L., Tuomilehto, J., & Holman, R. R. (2021). Predicting the risk of developing type 2 diabetes in Chinese people who have coronary heart disease and impaired glucose tolerance. J Diabetes, 13(10), 817-826. https://doi.org/10.1111/1753-0407.13175
50. Yan, F., Cha, E., Lee, E. T., Mayberry, R. M., Wang, W., & Umpierrez, G. (2016). A Self-assessment Tool for Screening Young Adults at Risk of Type 2 Diabetes Using Strong Heart Family Study Data. Diabetes Educ, 42(5), 607-617. https://doi.org/10.1177/0145721716658709
51. Yeh, H.-C., Duncan, B. B., Schmidt, M. I., Wang, N.-Y., & Brancati, F. L. (2010). Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Annals of Internal Medicine, 152(1), 10-17.
52. Zand, A., Ibrahim, K., & Patham, B. (2018). Prediabetes: Why Should We Care? Methodist Debakey Cardiovasc J, 14(4), 289-297. https://doi.org/10.14797/mdcj-14-4-289
About the Authors
P. WongrithThammasart University; Walailak University
Thailand
Paleeratana Wongrith - MSc (Health Education & Behavioral Science), Doctoral candidate, Assistant Professor
222 Thaiburi, Thasala District Nakhonsrithammarat, Thailand, 80160
Competing Interests:
No declare
S. Dangkrajang
Thailand
Suphika Dangkrajang - ED.D., Assistant Professor.
Bangkok
Competing Interests:
No declare
T. T. Nam
Viet Nam
Truong Thanh Nam - PhD, Assistant Professor.
Can Tho
Competing Interests:
No declare
Supplementary files

|
1. Appendix | |
| Subject | ||
| Type | Исследовательские инструменты | |
Download
(85KB)
|
Indexing metadata ▾ | |
|
|
2. Figure 1. PRISMA 2020 flow diagram. | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(696KB)
|
Indexing metadata ▾ | |
Review
For citations:
Wongrith P., Dangkrajang S., Nam T. Diabetes Risk Screening Tools for Prediabetes: A Comprehensive Scoping Review of Evidence and Implementation. Diabetes mellitus. 2025;28(4):348-358. https://doi.org/10.14341/DM13324

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0).















































