Scroll to:
The Role of Thiosulfate Sulfurtransferase in Oxidative Stress for Type 2 Diabetes Mellitus
https://doi.org/10.14341/DM13279
Abstract
BACKGROUND: Type 2 diabetes mellitus (T2DM) is associated with oxidative stress, leading to insulin resistance. Thiosulfate sulfurtransferase (TST) is mitochondrial enzyme involved in the reaction with cyanide, endogenous hydrogen sulfide (H₂S), and reactive oxygen species.
AIM: This study aimed to investigate the relationship between TST enzyme and both oxidative and anti-oxidative stress markers in T2DM patients. TST is believed to be related to oxidative stress, which plays a crucial role in determining the severity and progression of the disease.
MATERIALS AND METHODS: A case-control study included (150) T2DM patients who were taking the drug Metformin (Glucophage) 500 mg twice daily as well as (150) healthy subjects aged between 33 to 65 years. TST activity was estimated based on the sulfur transfer and thiocyanate formation. Malonaldehyde (MDA), peroxynitrite, peroxidase, aryl esterase, vitamin C, vitamin E, thioredoxin (Trx) and glutathione (GSH) were also measured. In addition to clinical markers, all measurements were made in two replicates, statistical analyses were conducted, and data were presented as a median and interquartile range.
RESULTS: TST activity was significantly lower (by 55%) in T2DM patients compared to the controls (8.5 (3.8) vs. 19 (2) U/ml, respectively). There was an inverse relationship between enzyme activity and age, whereas enzyme activity increased with smoking. Antioxidant compounds such as vitamin C, vitamin E, GSH, Trx, and arylesterase activity were significantly lower, while oxidant markers including peroxidase activity, MDA, and peroxynitrite, were significantly higher. TST activity showed a negative correlation with MDA and peroxynitrite, and a positive correlation with Trx and GSH.
CONCLUSION: TST activity is reduced in T2DM patients and is associated with oxidative stress. This suggest that TST may play a protective role against oxidative stress, making it a potential indicator of metabolic regulation and a possible therapeutic target.
For citations:
Mawajdeh M.M., Allwsh T.A. The Role of Thiosulfate Sulfurtransferase in Oxidative Stress for Type 2 Diabetes Mellitus. Diabetes mellitus. 2025;28(4):359-366. https://doi.org/10.14341/DM13279
BACKGROUND
Type 2 diabetes mellitus (T2DM) is the most comm on type of diabetes and accounts for the highest incidence. Diabetes is rapidly emerging as a critical global health concern, affecting approximately 529 million individuals aged between 20 and 79 in 2021, with this number expected to rise to 783 million by 2045 [1]. T2DM occurs when the body’s cells become resistant to the effects of insulin or when the pancreas fails to produce enough insulin to regulate glucose levels within the normal range. Diabetes is also associated with lifestyle factors such as physical inactivity and weight gain due to an unhealthy diet [2]. Oxidative stress (OS) plays a pivotal role in the pathophysiology of T2DM [3]. Chronic hyperglycemia in T2DM promotes ROS production, which adversely affects insulin-producing pancreatic beta cells due to their limited antioxidant capacity. This oxidative stress impairs beta-cell function, reduceing insulin secretion and increasing insulin resistance [4]. This imbalance also affects GLUT-4 regulation, further contributing to insulin resistance [5] and playing a significant role in diabetes-related complications, including cardiovascular diseases [6].
Thiosulfate sulfurtransferase (TST), also known as Rhodanese (EC 2.8.1.1) was first identified as an enzyme involved in cyanide detoxification. It is essential for degradation of reactive oxygen species (ROS) and metabolism of sulfide (H2S) by sulfur dioxygenase’s [7]. The activity of TST has been linked to metabolic diseases through its role in stimulating mitochondrial activity and increasing antioxidants level. It also plays a significant role in decreasing H2S levels [8]. Therefore, TST is considered a crucial enzyme for protecting cells from oxidative stress and supporting metabolic stability. It achieves this by neutralizing reactive oxygen and nitrogen species (ROS and RNS), helping to maintain redox balance [9]. Additionally, it supports antioxidant systems, particularly by regenerating GSH and Trx, which are essential for protecting cells, especially mitochondria, from oxidative damage [10]. Beyond its role as an antioxidant, TST regulates hydrogen sulfide signaling and redox balance, further contributing to cell protection [11]. Hydrogen peroxide (H₂O₂) oxidizes TST at its active site, which contains the amino acid cysteine, and this reaction can be reversed by Trx [12].
TST is also involved in the formation and repair of iron-sulfur clusters, which are critical for the functioning of various mitochondrial enzymes, including those involved in the electron transport chain (ETC). It facilitates the restoration of these clusters by donating sulfur atoms, thereby indirectly supporting mitochondrial respiration and cellular energy production [13]. Moreover, TST interacts with enzymes involved in oxidative metabolism, such as xanthine oxidase, succinate dehydrogenase, and NADH dehydrogenase, highlighting its pivotal role in regulating cellular metabolism and protecting against oxidative stress [14].
However, studies [14–15] have shown that the understanding of the role of TST in response to oxidative stress is unclear, and the mechanism by which TST acts on mitochondrial superoxide remains unknown. Nevertheless, the possibility remains that this could be harnessed to protect cells in vivo from oxidative damage.
RESEARCH AIM
The study was conducted on TST in individuals with T2DM (at the Al-Wafa Center for Endocrinology and Diabetes in Mosul) and its relationship to oxidative stress. The study involved estimating oxidizing and antioxidant compounds, including MDA, peroxynitrite, peroxidase, aryl esterase, vitamin C, vitamin E, Trx, and GSH. The study hypothesized that TST related to oxidative stress plays a crucial role in determining the severity and progression of the disease and could serve as a potential therapeutic target.
MATERIALS AND METHODS
Place of the research. Biochemistry laboratories in the Department of Chemistry, Collage of Science, University of Mosul.
Period of the research. From December 2023 to April 2024
STUDY DESIGN
The study design is a single-center, observational, case-control, and cross-sectional study. It included two groups:
Patients: 150 individuals with T2DM, 85 males and 65 females, aged 33-65 years. All patients were visiting Al-Wafa Endocrinology and Diabetes Center in Mosul, Iraq. The patients were diagnosed and followed up by specialized physicians, and all patients were using the drug Metformin (Glucophage) 500 mg twice daily. Patient information was collected through questionnaires and interviews with patients.
The Control: 150 healthy individuals who were not infected and showed no clinical symptoms, including 83 males and 67 females, matched in age to the patients.
Physical examinations were performed, and demographic data such as systolic and diastolic blood pressures, hip and waist circumference (in cm) and body mass index (BMI) [weight (kg)/height (m²)] were measured for all participants.
Exclusion Criteria: Patients with diabetic nephropathy, type 1 diabetes, gestational diabetes, or any hormonal abnormalities were excluded.
LABORATORY PARAMETER ASSESSMENTS
This research was conducted at the Chemistry Department, College of Science, University of Mosul. Five milliliters of venous blood were collected from the study participants after at least 8 hours of overnight fasting. The blood biomarker was assessed using a commercial kit according to the manufacturer’s instructions. The basic principles of estimation methods:
The HbA1c test is based on a colorimetric enzymatic assay. The insulin was estimated using electrochemiluminescence immunoassay (ECLIA) based on the Sandwich principle with kits from Roche Diagnostics Corporation, utilizing the Cobas e 411 analyzer.
Glucose, uric acid, albumin, and lipid profile (total cholesterol, triglycerides, and HDL) were also measured. Additionally, urea and creatinine levels were measured to evaluate renal function, and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were assessed for liver function, using an enzymatic colorimetric method with a kit from Biolabo SAS (France, Catalog No. 02160).
Glucose was measured using the Trinder method. Triglycerides were broken down by lipase into fatty acids and glycerol. Total cholesterol (TC) was measured based on its hydrolysis into free cholesterol and fatty acids. The sedimentation method was used to estimate the HDL-C , and albumin was measured using the bromocresol green method. Creatinine was measured using the Jaffe method, urea was measured using the urease and uric acid was measured using the uricase. The activity of (GPT or ALT) and (GOT or AST) catalyze the amino conversion reaction to form a colored compound under certain conditions. Very-low-density lipoprotein cholesterol (VLDL) was calculated by dividing triglycerides (TG) by 2.5, while low-density lipoprotein cholesterol (LDL) was determined using the Friedwald formula.
The HOMA-IR was calculated as = [Fasting Insulin (μU/ml) ∗ fasting glucose (mmol/L)] / 22.5 [16]
In the next set of experiments conducted using manual methods, the activity of TST was estimated according to the method of (Urbanska et al., 2002) [17]. TST catalyzes the transfer of a sulfur atom from the sulfur donor (thiosulfate) to nucleophilic acceptor (cyanide). The levels of MDA, an oxidative stress biomarker, were measurd using a method based on reaction of MDA with thiobarbituric acid (TBA) to produce a colored compound [18]. Trx was determined based on its ability to reduce disulfide bonds in insulin, using dithiothreitol as a reducing agent [19]. According to [20], peroxidase activity was estimated based on the enzymatic oxidation of hydrogen peroxide, forming a colored substance. Additionally, arylesterase activity was measured using a method in which the enzyme breaks down phenyl acetate into phenol and acetic acid. GSH was estimated using a modified Ellman’s reagent, which contains 5,5’-dithiobis-(2-nitrobenzoic acid), and reacts with the sulfhydryl (-SH) group in GSH to form a yellow compound. Vitamin E (alpha-tocopherol) was estimated based on oxidation-reduction reactions known as Emmeric-Engle Reaction.
DATA ANALYSIS
The Statistical Package for the social Sciences (SPSS-2022) was used to analyse the study’s data and to obtain the median and interquartile range. A t-test was used to compare the patients to controls. To determine a linear relationship between various parameters, the Pearson correlation coefficient (r) was used. A P-value ≤0.05 was considered statistically significant.
ETHICS REVIEW
The study adhered to all legal and ethical standards and requirements. Approval was obtained from the Ministry of Health/Nineveh Health Directorate, Mosul, Iraq (Protocol Number: 2023228). Written informed consent was obtained from all participants, and the consent forms were signed on December 13, 2023
RESULTS
A total 150 individuals with T2DM were included in the case group (Female/Male: 85/65, mean age: 50 (8.5) years), and 150 healthy individuals without diabetes were included in the control group (Female/Male: 83/67, mean age: 56.5 (10.5) years. Demographic and anthropometric characteristics of the study population are shown in Table 1. Systolic and diastolic blood pressure, waist circumference, and hip circumference were significantly higher in the T2DM group compared to the control group, while age, sex, and smoking habits were similar between the groups. In the comparison of glucose, total cholesterol, triglycerides (TG), LDL-C, VLDL-C, and Non-HDL-C indices, thes were found to be statistically significantly higher in the T2DM group, while HDL-C levels were lower (Table 1). Urea and creatinine levels for renal function, AST, and ALT for liver function showed no statistical difference between T2DM and the control group, as did uric acid and albumin levels.
Table 1. The demographic and anthropometric values of the participants
|
Variable |
Controls group Median & (IQR) |
T2DM group Median & (IQR) |
reference values |
|
Age (year) |
50 (8.5) |
56.5 (10.5) |
- |
|
Sex (Female / Male) |
83/67 |
85/65 |
- |
|
Smoking/ non-Smoking |
80/70 |
82/68 |
- |
|
Systolic blood pressure (mmHg) |
121 (11) |
144 (14)* |
<120 |
|
Diastolic blood pressure (mHg) |
70 (10) |
81 (10)* |
<80 |
|
weight (Kg) |
77 (20) |
98 (33)* |
- |
|
BMI (Kg/m²) |
34 (10.5) |
38.5 (11) * |
30 and 39.9 obesity. |
|
Waist Circumference (cm) |
93 (12) |
127 (14.5)* |
80–94 |
|
Hip Circumference (cm) |
104.5 (12) |
118.5 (10)* |
94–108 |
|
Glucose (mmol/L) |
5.4 ± (1.2) |
10 (2.9)* |
3.9–5.6 |
|
HbA1c % |
4.5(1.9) |
7.3 (2.7)* |
4–5.6 |
|
TC (mmol/L) |
1.1 (0.55) |
3 (1)* |
Less than 5.2 |
|
TG (mmol/L) |
0.82 (0.25) |
1.3 (0.35)* |
Less than 1.7 |
|
HDL (mmol/L) |
7.0 (0.6) |
2.5 (0.5)* |
1.6 |
|
LDL (mmol/L) |
0.7 (0.2) |
1.6 ( 0.3 )* |
Less than 2.6 |
|
VLDL (mmol/L) |
0.085 (0.038) |
0.223 (0.085)* |
0.05 to 0.78 |
|
Non HDL (mmol/L) |
4.1 (0.8) |
6.8 (0.6)* |
Less than 4mmol/L |
|
Urea (mg/dL) |
27 (3.2) |
26.8 (2.5) |
20–45 |
|
Creatinine (mg/dL) |
0.72 (0.06) |
0.79 (0.05) |
0.7 to 1.3 |
|
AST (U/L) |
23.8 (4) |
27 (6) |
8–48 |
|
ALT (U/L) |
24.5 (2.9) |
28 (4) |
7–55 |
|
Albumin(g/L) |
42 (4) |
39 (3. 5) |
34–54 |
|
Uric acid(mg/dl) |
3.9 (0.3) |
4.7 (0.5) |
2.7–7.3 |
|
Insulin (µU/mL) |
9.5 (1.7) |
7.6 (1.2)* |
5–12 |
|
HOMA-IR |
1.7 (0.5) |
2.1 (0.3)* |
0.5–1.4 |
*Significant at the level P≤0.01
Note: HbA1C : Hemoglobin A1C ; BMI: Body mass index ; TG: Triglyceride; HDL: High Density Lipoprotein; Non HDL: non- High Density Lipoprotein ; TC: Total cholesterol; LDL: Low Density Lipoprotein; VLDL: Very-low-density lipoprotein; AST: Aspartate Transaminase; ALT: Alanine Transaminase.
Serum TST activity was lower in T2DM cases than in the healthy control group (8.5(3.8) vs. 19(2) U/ml, respectively) as shown in (Table 2). There was a 46% decrease in the diabetic group compared to the control group, with a statistically significant variation (p<0.001), this finding is consistent with what was reportd by Kruithof et al., 2022 [14], who found that TST is a genetic marker for resisting obesity-related T2DM due to a negative genetic association between them. It was also found that enzyme activity decreased with increasing age, indicating an inverse relationship between enzyme activity and age. However, no statistical difference was observed in TST activity between females and males in the groups. Additionally, it was found that enzyme activity increased with smoking.
Table 2. TST Activity of Control and Patient Groups
|
Influential factors |
Activity of TST (U /ml) Median & (IQR) |
% |
||
|
control group |
(T2D) Patients group |
|||
|
Age (years) |
(35–45) |
24 (6)a |
14 (4)a |
-41 |
|
(46–55) |
19 (3)b |
10 (2)b* |
-47 |
|
|
(>55) |
14 (2)c |
6 (3)c* |
-57 |
|
|
Sex |
Male |
20(3)a |
10 (1)a* |
-50 |
|
Female |
23 (3)a |
12 (3)a* |
-47 |
|
|
Smoking |
Smoking |
24 (4)a |
14 (3)a* |
-41 |
|
Non-smoking |
16 (3)b |
6 (2)b* |
-62 |
|
|
Total |
19 (2) |
8.5 (3.8) |
- 55 |
|
* Indicates the statistical difference at the level p ≤ 0.01 between the patients and the control group.
Different letters: indicate the presence of a statistical difference between the subgroups of the same group, while similar letters indicate the absence of a statistical difference at the level p ≤ 0.01.
The antioxidant compounds, such as vitamin C, vitamin E, GSH, Trx, and the activity of arylesterase, were significantly lower in T2DM compared to the control group, as shown in Table 3. On the other hand, oxidant compounds, such as the activity of peroxidase, MDA, and peroxynitrite, were significantly higher in T2DM.
Table 3. The oxidant and antioxidant compounds for the control and patient groups
|
The oxidant and antioxidant compounds |
Control group Median & (IQR) |
(T2D) Patients group Median & (IQR) |
|
MDA (µmol/L) |
2.7 (2) |
9.2 (3)* |
|
Peroxynitrite(M\L) |
16.5 (4) |
52 (5)* |
|
Peroxidase(U/L) |
71(10) |
164 (20)* |
|
Trx (µmol/L) |
5.6 (2) |
2.3 (1.5) * |
|
GSH (µmol/L) |
13 (3) |
4.8 (2)* |
|
Arylesterase(U/L) |
108 (10) |
73 (11) * |
|
E (µmol\L) vitamin |
38 (4) |
20 (5)* |
|
vitamin C (mg\dl) |
6.5 (3) |
3.4 (2)* |
*Significant at the level P≤0.01
Note: MDA: malondialdehyde; GSH: glutathione; Trx: Thioredoxin.
Table 4 and Figure 1 show the correlation coefficient of TST activity with antioxidant and oxidant compounds in the T2DM group. The results of the correlation coefficient showed a positive correlation between TST activity and both (Trx) and (GSH) (r=0.75, r=0.77, respectively), and a negative correlation between TST activity and both MDA and peroxynitrate (r=-0.73, r=-0.81, respectively).
Table 4. The correlation between TST activity and the oxidants and antioxidants compounds for patient group
|
TST activity |
|
|
The oxidants and antioxidants compounds |
R-value |
|
MDA (µmol/L) |
-0.73* |
|
Peroxynitrite (M/L) |
-0.81* |
|
Peroxidase(U/L) |
0.26 |
|
Trx (µmol/L) |
0.75* |
|
GSH (µmol/L) |
0.77* |
|
Arylesterase (U/L) |
0.38 |
|
vitamin C (mg/dl) |
0.25 |
|
vitamin E (µmol/L) |
0.41 |
*Significance at p≤0.01
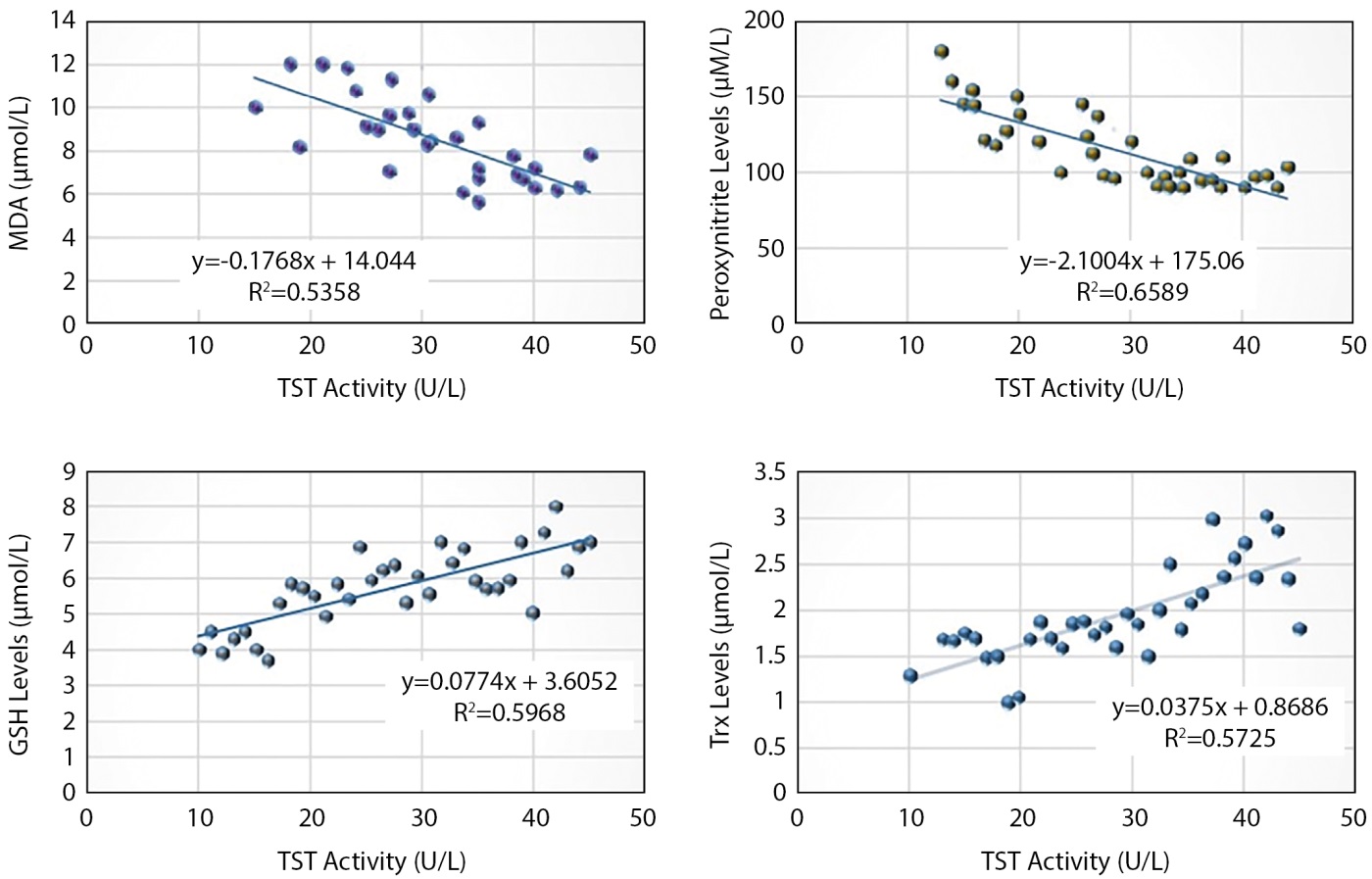
Figure 1. The correlation of TST activity with MDA ,Peroxynitrite, Trx and GSH
DISCUSSION
The results of the study showed that the activity of TST decreases in T2DM patients who visit the Al-Wafa Center for Endocrinology and Diabetes in Mosul, aged 33 to 65 years, and for both sexes, compared to the healthy group. Additionally, advancing age is inversely related to TST activity, while activity does not differ between males and females. TST activity also increases in smokers.
According to a study by Perridon et al. [21] on decreased TST activity, the reason may be the loss of proteostasis, characterized by a reduced ability to maintain protein quality control in T2DM, which could result in the misfolding or degradation of TST, decreasing its levels. Moreover, stem cell exhaustion, which reduces the regenerative capacity of stem cells, may lead to fewer cells overall, including those that produce TST. Consequently, the deficiency of TST in aging individuals could stem from the combined effects of these interconnected hallmarks of aging, leading to reduced production or increased degradation of the enzyme [22].
Low TST activity in individuals with diabetes can be attributed to several factors linked to the disease. Diabetes can lead to mitochondrial dysfunction, which impairs TST activity. Additionally, diabetes is associated with elevated oxidative stress, which can damage mitochondrial enzymes like TST. The increased generation of reactive oxygen species (ROS) in diabetes can directly inhibit TST activity [9]. TST is involved in sulfur metabolism, and disturbances in this pathway, which are prevalent in diabetes, can reduce enzyme expression and functionality [15].
The decline in physiological functions and cellular damage associated with aging could impact enzymes involved in critical metabolic pathways, including TST. Cellular senescence, which results in the accumulation of senescent cells that secrete factors disrupting normal cellular function, can affect enzyme production [22]. Additionally, telomere attrition, where shortened telomeres lead to cellular senescence or apoptosis, may reduce the number of cells capable of producing TST. Age-related epigenetic alterations can further down-regulate TST production by changing gene expression regulation [21]. Smoking increases oxidative stress, and TST activity may be elevated in smokers due to the increased need to convert cyanide to thiocyanate as part of the smoking detoxification process [23]. Therefore, it can be concluded that smoking may negatively affect the enzyme pathway in metabolic processes and shift the balance toward the cyanide detoxification pathway.
Many studies have shown that increased serum levels of MDA are due to enhanced oxidative stress processes in patients, which lead to an increase in the levels of free radicals and lipid peroxides, and, thus, an increase in the concentration of MDA, which is their metabolic product [24–25]. Peroxynitrite and peroxidase levels were increased due to oxidative stress in diabetes, as these are among the oxidizing factors whose effectiveness increases in the presence of oxidative stress, which causes the development of insulin resistance, dysfunction of pancreatic beta cells, mitochondrial dysfunction, and complications of diabetes [26]. Peroxynitrite is a strong oxidizing substance formed by the combination of NO with O2−• and decomposes quickly into highly reactive oxidizing species [27–28]. Peroxynitrite production increases significantly in many disorders due to increased oxidative stress, which leads to necrosis and programmed cell death [29]. Peroxynitrite can react with protein tyrosine lipoproteins and residues to form 3-nitrotyrosine. Elevated levels of 3-nitrotyrosine are considered a marker of inflammation in patients with T2DM. Peroxynitrite might play a role in both macrovascular and microvascular damage in T2DM [30].
Many studies have shown that decreased serum levels of Trx in patients with T2DM may be due to increased oxidative stress, which leads to increased production of free radicals and an increase in the concentration of thioredoxin-interacting protein (TXNIP). TXNIP increases when glucose concentration rises, leading to the generation of insulin resistance. Trx improves β-cell survival under a variety of environmental stresses [31–32]. Trx overexpression delays the onset of diabetes and protects against β-cell cytotoxicity [33–34].
GSH is a powerful antioxidant that scavenges free radicals, reduces fat peroxidation, and decreases the percentage of oxidized LDL. Therefore, the concentration of GSH decreases due to its consumption or may be due to a decrease in the activity of glutathione peroxidase (GPx) [35]. Reduced serum arylesterase activity in diabetic patients might be caused by heightened oxidative stress, decreased HDL-C levels, and elevated BMI [36-37]. The reason for its decrease is due to the binding of fats to the free sulfhydryl group, rendering them ineffective [38].
Low levels of vitamins C and E may impair the function of beta cells in the pancreas, leading to insulin resistance and high blood sugar [39]. It has been found that hyperglycemia was significantly negatively associated with vitamins C and E in diabetics. Meanwhile, MDA was significantly inversely related to vitamins C and E. Additionally, oxidative stress in T2DM is indicated by reduced serum levels of vitamins C and E [40].
The results showed a negative correlation between TST activity with MDA and peroxynitrite in the T2DM group. The reason for this may be that TST deficiency leads to increased ROS production in fat cells, which may be associated with elevated MDA levels [41]. TST deficiency also affects the production of reactive nitrogen species (RNS), including peroxynitrite. Low TST activity alters the balance of RNS, contributing to an impaired ability to manage oxidative damage. Peroxynitrite is highly reactive and can cause damage to proteins, lipids, and DNA, exacerbating oxidative stress [9].
In addition, the results showed a positive correlation in the activity of the TST in the T2DM group with GSH and Trx. GSH plays a crucial role in modulating the activity of this enzyme. Involved in TST, GSH acts as an essential cofactor that helps maintain the redox state of the cell, thus supporting the proper function of enzymes. The interaction between GSH and TST is critical for maintaining cellular detoxification, especially under oxidative stress [42].
Trx, due to its redox modulatory function, can indirectly influence TST activity by maintaining a balanced redox state, which is critical for cysteine-dependent enzymatic functions, such as TST. This may contribute to maintaining a cellular environment that allows optimal Trx function, particularly in detoxifying reactive sulfur species that can arise under oxidative conditions. Together, Trx and GSH contribute to cellular defense mechanisms against oxidative damage [43].
The results of the study show that decreased TST activity is associated with increased oxidative stress in T2DM patients, reflecting the biological importance of TST. Its activity can be enhanced by thiosulfate, which reacts with the thiol group to generate hydrogen sulfide, therapy removing oxidative radicals and increasing GSH levels. Additionally, thiosulfate acts as an antioxidant by eliminating superoxide. TST may play an important role in protecting cells from oxidative stress and supporting metabolic stability through three possible mechanisms, firstly: Neutralizing reactive oxygen and nitrogen species (ROS and RNS): This helps maintain redox balance and supports antioxidant systems, particularly by regenerating GSH and Trx, which are essential for protecting cells especially mitochondria from oxidative damage. Secondly: Regulating hydrogen sulfide signaling and redox balance: This further contributes to cellular protection. Finally: Interacting with enzymes involved in oxidative metabolism: By doing so, TST helps regulate cellular metabolism and protect against oxidative stress.
LIMITATIONS OF THE RESEARCH
The study had some limitations. As a case-control study conducted at a single medical center, the results may have been influenced by the patients’ lifestyle. Additionally, the study included specific age criteria, so the findings may not be applicable to all patients across different age groups. A multicenter study with a larger sample size, including individuals over the age of sixty-five, is needed to validate these results.
CONCLUSION
The activity of TST is low in Type 2 diabetic patients and is correlated with MDA and peroxynitrite levels as oxidants, as well as Trx and GSH as markers of antioxidants. These findings suggest that negative changes in the role of TST, associated with increased oxidative stress, may contribute to the progression and severity of T2DM. This indicates that TST could serve as a key biomarker in the development and progression of oxidative stress processes. Furthermore, regulating these processes by enhancing enzyme activity may present a potential therapeutic target. Future research involving different groups and larger scales is recommended to validate and expand upon these findings.
ADDITIONAL INFORMATION
Source of financing. None.
Conflicts of interest. None.
Participation of authors. Marwa M. Mawajdeh — carried out all the experiments, obtained and analyzed the data, interpreted the results, and wrote the article. Thikra Ali Allwsh — made a significant contribution to the study design, obtained and analyzed the data, interpreted the results, and wrote the article. All the authors approved the final version of the article before publication and expressed their consent to be responsible for all aspects of the work, including proper investigation and resolution of issues related to the accuracy or integrity of any part of the work.
Acknowledgments. We would like to express our gratitude and appreciation to Al-Wafa Center for Endocrinology and Diabetes in Mosul for helping us work with all patients, as well as to the University of Mosul for supporting the completion of the study.
References
1. International Diabetes Federation. (2023). IDF Diabetes Atlas (10th ed.). International Diabetes Federation. https://diabetesatlas.org
2. Galicia-Garcia, U., Benito-Vicente, A., Jebari, S., Larrea-Sebal, A., Siddiqi, H., Uribe, K. B., ... & Martín, C. (2020). Pathophysiology of type 2 diabetes mellitus. International journal of molecular sciences, 21(17), 6275.
3. Vona, R., Pallotta, L., Cappelletti, M., Severi, C., & Matarrese, P. (2021). The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants (Basel, Switzerland), 10(2), 201. https://doi.org/10.3390/antiox10020201
4. Pasupuleti, V. R., Arigela, C. S., Gan, S. H., Salam, S. K. N., Krishnan, K. T., Rahman, N. A., & Jeffree, M. S. (2020). A Review on Oxidative Stress, Diabetic Complications, and the Roles of Honey Polyphenols. Oxidative medicine and cellular longevity, 2020, 8878172. https://doi.org/10.1155/2020/8878172
5. Yaribeygi, H., Sathyapalan, T., Atkin, S. L., & Sahebkar, A. (2020). Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxidative medicine and cellular longevity, 2020, 8609213. https://doi.org/10.1155/2020/8609213
6. Charlton, A., Garzarella, J., Jandeleit-Dahm, K. A., & Jha, J. C. (2020). Oxidative stress and inflammation in renal and cardiovascular complications of diabetes. Biology, 10(1), 18.
7. Chaudhary M, Gupta R. Cyanide detoxifying enzyme: Rhodanese. Curr. Biotechnol. e. 2012;1:327–335. doi: 10.2174/2211550111201040327. [CrossRef] [Google Scholar]
8. Morton, N., Beltram, J., Carter, R. et al. Genetic identification of thiosulfate sulfurtransferase as an adipocyte-expressed antidiabetic target in mice selected for leanness. Nat Med 22, 771–779 (2016). https://doi.org/10.1038/nm.4115[PMC free article] [PubMed] [CrossRef] [Google Scholar]
9. Al-Dahmani ZM, Hadian M, Ruiz-Moreno AJ, Maria S-GA, Batista FA, Zhang R, et al. Identification and characterization of a small molecule that activates thiosulfate sulfurtransferase and stimulates mitochondrial respiration. Protein Science. 2023; 32(11):e4794. https://doi.org/10.1002/pro.4794
10. Kruithof, P. D., Lunev, S., Lozano, S. P. A., de Assis Batista, F., Al-Dahmani, Z. M., Joles, J. A., ... & van Goor, H. (2020). Unraveling the role of thiosulfate sulfurtransferase in metabolic diseases. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1866(6), 165716.
11. https://doi.org/10.1016/j.bbadis.2020.165716.
12. Nandi, D. L., Horowitz, P. M., & Westley, J. (2000). Rhodanese as a thioredoxin oxidase. The international journal of biochemistry & cell biology, 32(4), 465–473. https://doi.org/10.1016/s1357-2725(99)00035-7
13. Urbanska, K., Wiater, A., & Nowak, A. (2002). Thiosulfate sulfurtransferase activity in rat tissues in the presence of organic nitriles. Acta Biochimica Polonica, 49(1), 109-114.
14. Saadon, S.R., & Allwsh, T.A. (2024). A CLINICAL STUDY OF LIPOCALIN 2 AND ITS RELATION WITH OXIDATIVE AND ANTIOXIDATIVE FACTORS IN ARTHRITIS. MMSL, 93(4), 335-341. doi: 10.31482/mmsl.2023.040.
15. Arnér, E. S. J., L. Zhong, A. Holmgren, and P. Lester. (1999). Preparation and assay of mammalian thioredoxin and thioredoxin reductase. Methods Enzymol. 300:226-239. https://doi.org/10.1016/S0076-6879(99)00129-9.
16. Perridon, B. W., Leuvenink, H. G., Hillebrands, J. L., van Goor, H., & Bos, E. M. (2016). The role of hydrogen sulfide in aging and age-related pathologies. Aging, 8(10), 2264–2289. https://doi.org/10.18632/aging.101026
17. Szlęzak, D., Bronowicka-Adamska, P., Hutsch, T., Ufnal, M., & Wróbel, M. (2021). Hypertension and Aging Affect Liver Sulfur Metabolism in Rats. Cells, 10(5), 1238. https://doi.org/10.3390/cells10051238
18. Luo, Y., Chatre, L., Melhem, S., Al-Dahmani, Z. M., Homer, N. Z. M., Miedema, A., Deelman, L. E., Groves, M. R., Feelisch, M., Morton, N. M., Dolga, A., & van Goor, H. (2023). Thiosulfate sulfurtransferase deficiency promotes oxidative distress and aberrant NRF2 function in the brain. Redox biology, 68, 102965. https://doi.org/10.1016/j.redox.2023.102965
19. San Gabriel, P. T., Liu, Y., Schroder, A. L., Zoellner, H., & Chami, B. (2020). The role of thiocyanate in modulating myeloperoxidase activity during disease. International Journal of Molecular Sciences, 21(17), 6450.
20. Mas-Bargues, C., Escriva, C., Dromant, M., Borras, C., & Vina, J. (2021). Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Archives of biochemistry and biophysics, 709, 108941.
21. Najafi, A., Pourfarzam, M., & Zadhoush, F. (2021). Oxidant/antioxidant status in Type-2 diabetes mellitus patients with metabolic syndrome. Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences, 26, 6. https://doi.org/10.4103/jrms.JRMS_249_20
22. Newsholme, P., Keane, K. N., Carlessi, R., & Cruzat, V. (2019). Oxidative stress pathways in pancreatic β-cells and insulin-sensitive cells and tissues: importance to cell metabolism, function, and dysfunction. American Journal of Physiology-Cell Physiology, 317(3), C420-C433.
23. Bahadoran, Z., Mirmiran, P., Kashfi, K., & Ghasemi, A. (2023). Vascular nitric oxide resistance in type 2 diabetes. Cell Death & Disease, 14(7), 410.
24. Piacenza, L., Zeida, A., Trujillo, M., & Radi, R. (2022). The superoxide radical switch in the biology of nitric oxide and peroxynitrite. Physiological Reviews, 102(4), 1881-1906.
25. Bala, A. (2024). Regulatory role of peroxynitrite in advanced glycation end products mediated diabetic cardiovascular complications. World Journal of Diabetes, 15(3), 572.
26. Tuell, D., Ford, G., Los, E., & Stone, W. (2024). The Role of Glutathione and Its Precursors in Type 2 Diabetes. Antioxidants (Basel, Switzerland), 13(2), 184. https://doi.org/10.3390/antiox13020184
27. Stancill, J. S., & Corbett, J. A. (2021). The role of thioredoxin/peroxiredoxin in the β-cell defense against oxidative damage. Frontiers in Endocrinology, 12, 718235.
28. Xu, L. L., Gao, W., Chen, Z. M., Shao, K. K., Wang, Y. G., Cui, L. L., & Guo, N. Z. (2020). Relationships between diabetic nephropathy and insulin resistance, inflammation, Trx, Txnip, CysC and serum complement levels. European Review for Medical & Pharmacological Sciences, 24(22).
29. Kar, A., Paramasivam, B., Jayakumar, D., Swaroop, A. K., & Jubie, S. (2023). Thioredoxin Interacting Protein Inhibitors in Diabetes Mellitus: A Critical Review. Current Drug Research Reviews Formerly: Current Drug Abuse Reviews, 15(3), 228-240.
30. Liu, C., Dong, W., Lv, Z., Kong, L., & Ren, X. (2022). Thioredoxin-interacting protein in diabetic retinal neurodegeneration: A novel potential therapeutic target for diabetic retinopathy. Frontiers in neuroscience, 16, 957667. https://doi.org/10.3389/fnins.2022.957667
31. Averill-Bates, D. A. (2023). The antioxidant glutathione. In Vitamins and hormones (Vol. 121, pp. 109-141). Academic Press.
32. Nikzad, A., Alizadeh, A., Abediankenari, S., Kashi, Z., & Mahrooz, A. (2023). Paraoxonase 1 activity is associated with interleukin-6 levels in type 2 diabetes: Effects of age and gender. International Journal of Preventive Medicine, 14(1), 23.
33. Castañé, H., Jiménez-Franco, A., Martínez-Navidad, C., Placed-Gallego, C., Cambra-Cortés, V., Perta, A. M., ... & Joven, J. (2023). Serum Arylesterase, Paraoxonase, and Lactonase Activities and Paraoxonase-1 Concentrations in Morbidly Obese Patients and Their Relationship with Non-Alcoholic Steatohepatitis. Antioxidants, 12(12), 2038.
34. Marsillach, J., Richter, R. J., Costa, L. G., & Furlong, C. E. (2021). Paraoxonase-1 (PON1) Status Analysis Using Non-Organophosphate Substrates. Current protocols, 1(1), e25. https://doi.org/10.1002/cpz1.25
35. Razip, N. N. M., Gopalsamy, B., Abdul Mutalib, M. S., Chang, S. K., Abdullah, M. M. J. A., Azlan, A., Rejali, Z., & Khaza'ai, H. (2021). Correlation between Levels of Vitamins D3 and E in Type 2 Diabetes Mellitus: A Case-Control Study in Serdang, Selangor, Malaysia. Nutrients, 13(7), 2288. https://doi.org/10.3390/nu13072288
36. Hattiwale, S., Jargar, J. J., Hattiwale, H. R., Ahmed, M. M., & Nazeer, M. (2022). Status of non-enzymatic antioxidant vitamins (C and E) in patients either with type 2 diabete mellitus or hypertension alone and coexisted diabetes and hypertension. ACADEMIC JOURNAL.
37. Mc Fadden, C. E. (2018). Investigating the role of thiosulfate sulfurtransferase in adipose tissue dysfunction in obesity.
38. Melideo, S. L., Jackson, M. R., & Jorns, M. S. (2014). Biosynthesis of a central intermediate in hydrogen sulfide metabolism by a novel human sulfurtransferase and its yeast ortholog. Biochemistry, 53(28), 4739–4753. https://doi.org/10.1021/bi500650h
39. Libiad, M., Motl, N., Akey, D. L., Sakamoto, N., Fearon, E. R., Smith, J. L., & Banerjee, R. (2018). Thiosulfate sulfurtransferase-like domain-containing 1 protein interacts with thioredoxin. The Journal of biological chemistry, 293(8), 2675–2686. https://doi.org/10.1074/jbc.RA117.000826
About the Authors
M. M. MawajdehIraq
Marwa M. Mawajdeh - Master’s student in Biochemistry.
Mosul
Competing Interests:
None
T. A. Allwsh
Iraq
Thikra A. Allwsh - Professor, PhD in Biochemistry; ResearcherID: https://www.researchgate.net/profile/Thikra-Allwsh; Scopus Author ID: 57226087609.
Iraq, Mosul, Al-Zahraa Street, 41003
Competing Interests:
None
Supplementary files
|
|
1. Figure 1. The correlation of TST activity with MDA ,Peroxynitrite, Trx and GSH | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(632KB)
|
Indexing metadata ▾ | |
Review
For citations:
Mawajdeh M.M., Allwsh T.A. The Role of Thiosulfate Sulfurtransferase in Oxidative Stress for Type 2 Diabetes Mellitus. Diabetes mellitus. 2025;28(4):359-366. https://doi.org/10.14341/DM13279

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0).















































